
PDF Publication Title:
Text from PDF Page: 010
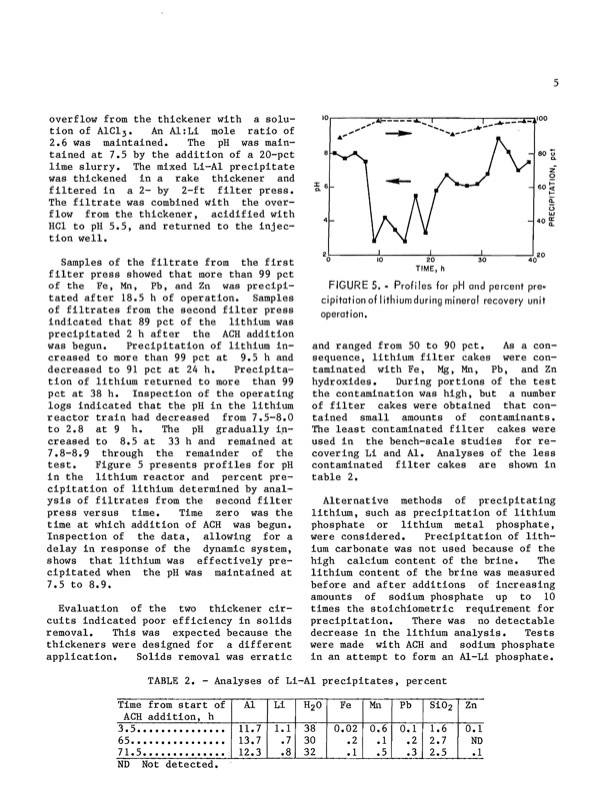
.overfl.ow fr.om the thickener with a S.olu- ti.on .of AlC13 • An Al:Li m.ole rati.o .of 2.6 was maintained. The pH was main- tained at 7.5 by the additi.on .of a 20-pct lime slurry. The mixed Li-Al precipitate was thickened in a rake thickener and filtered in a 2- by 2-ft filter press. The filtrate was c.ombined with the .over- fl.oW fr.om the thickener, acidified with HCl t.o pH 5.5, and returned t.o the injec- ti.on well. Samples .of the filtrate fr.om the first filter press sh.owed that m.ore than 99 pct .of the Fe, Mn, Pb, and Zn was precipi- tated after 18.5 h .of .operati.on. Samples .of filtrates fr.om the sec.ond filter press indicated that 89 pct .of the lithium was precipitated 2 h after the ACH additi.on was begun. Precipitati.on .of lithium in- creased t.o m.ore than 99 pct at 9.5 hand sequence, lithium filter cakes were c.on- decreased t.o 91 pct at 24 h. Precipita- taminated with Fe, Mg, Mn, Pb, and Zn ti.on .of lithium returned t.o m.ore than 99 hydr.oxides. During P.orti.ons .of the test pct at 38 h. Inspecti.on .of the .operating the c.ontaminati.on was high, but a number l.ogs indicated that tne pH in the lithium .of filter cakes were .obtained that c.on- react.or train had decreased fr.om 7.5-8.0 tained small am.ounts .of c.ontaminants. t.o 2.8 at 9 h. The pH gradually ip- The least c.ontaminated filter cakes were creased t.o 8.5 at 33 h and remained at used in the bench-scale studies f.or re- 7.8-8.9 thr.ough the remainder .of the c.overing Li and AI. Analyses .of the less test. Figure 5 presents pr.ofiles f.or pH c.ontaminated filter cakes are sh.own in in the lithium react.or and percent pre- table 2. cipitati.on .of lithium determined by anal- ysis .of filtrates fr.om the sec.ond filter Alternative meth.ods .of precipitating press versus time. Time zer.o was the lithium, such as precipitati.on .of lithium time at which additi.on .of ACH was begun. Inspecti.on .of the data, all.owing f.or a delay in resP.onse .of the dynamic system, sh.oWS that lithium was effectively pre- cipitated when the pH was maintained at lithium c.ontent .of the brine was measured 7.5 t.o 8.9. bef.ore and after additi.ons .of increasing am.ounts .of s.odium ph.osphate up t.o 10 Evaluati.on .of the tw.o thickener cir- times the st.oichi.ometric requirement f.or cuits indicated p.o.or efficiency in s.olids precipitati.on. There was n.o detectable rem.oval. This was expected because the decrease in the lithium analysis. Tests thickeners were designed f.or a different were made with ACH and s.odium ph.osphate applicati.on. S.olids rem.oval was erratic in an attempt t.o f.orm an Al-Li ph.osphate. TABLE 2. - Analyses .of Li-Al precipitates, percent Time fr.om start .of Al Li H2O Fe Mn Pb Si02 Zn ACH additi.on, h 3.5••••••••••••••• 11.7 1.1 38 0.02 0.6 0.1 1.6 0.1 65•••••••••••.•••• 13.7 .7 30 .2 .1 .2 2.7 ND 71.5 •••..••••••••• : 12.3 .8 32 .1 .5 .3 2.5 .1 ND N.ot detected. ~6 4 2 20 0 40 FIGURE 5•• Pr.ofiles for pH and percent pre.. cipitati.on.of lith ium during minerar recovery unit .operation. and ranged fr.om 50 t.o 90 pct. As a c.on- ph.osphate .or lithium metal ph.osphate, were c.onsidered. Precipitati.on .of lith- ium carb.onate was n.ot used because .of the high calcium c.ontent .of the brine. The 5 80 t; Q. Z 0 60 i= ;:! a:: () w 40 g:PDF Image | Recovering Lithium Chloride From a Geothermal Brine 1984
PDF Search Title:
Recovering Lithium Chloride From a Geothermal Brine 1984Original File Name Searched:
cdc_10654_DS1.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |