
PDF Publication Title:
Text from PDF Page: 010
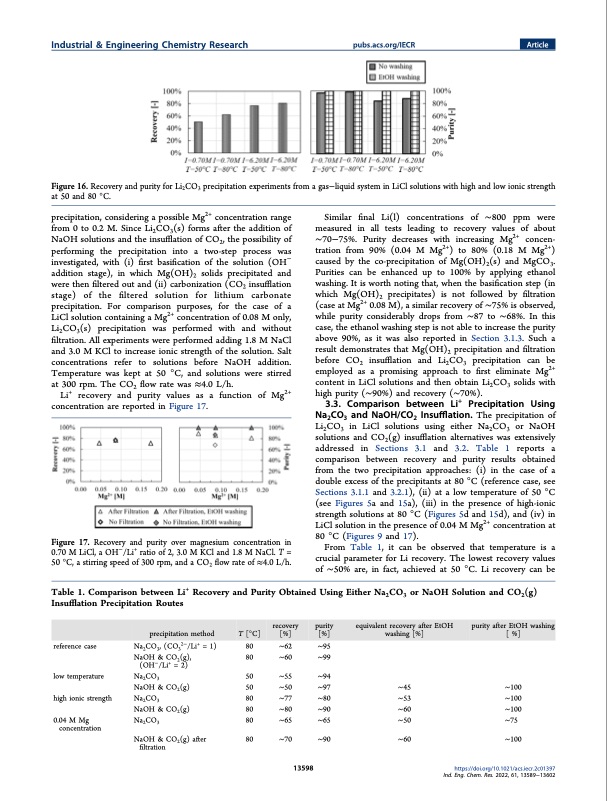
Industrial & Engineering Chemistry Research pubs.acs.org/IECR Article Figure 16. Recovery and purity for Li2CO3 precipitation experiments from a gas−liquid system in LiCl solutions with high and low ionic strength at 50 and 80 °C. precipitation, considering a possible Mg2+ concentration range from 0 to 0.2 M. Since Li2CO3(s) forms after the addition of NaOH solutions and the insufflation of CO2, the possibility of performing the precipitation into a two-step process was investigated, with (i) first basification of the solution (OH− addition stage), in which Mg(OH)2 solids precipitated and were then filtered out and (ii) carbonization (CO2 insufflation stage) of the filtered solution for lithium carbonate precipitation. For comparison purposes, for the case of a LiCl solution containing a Mg2+ concentration of 0.08 M only, Li2CO3(s) precipitation was performed with and without filtration. All experiments were performed adding 1.8 M NaCl and 3.0 M KCl to increase ionic strength of the solution. Salt concentrations refer to solutions before NaOH addition. Temperature was kept at 50 °C, and solutions were stirred at 300 rpm. The CO2 flow rate was ≈4.0 L/h. Li+ recovery and purity values as a function of Mg2+ concentration are reported in Figure 17. Figure 17. Recovery and purity over magnesium concentration in 0.70MLiCl,aOH−/Li+ ratioof2,3.0MKCland1.8MNaCl.T= 50 °C, a stirring speed of 300 rpm, and a CO2 flow rate of ≈4.0 L/h. Similar final Li(l) concentrations of ∼800 ppm were measured in all tests leading to recovery values of about ∼70−75%. Purity decreases with increasing Mg2+ concen- tration from 90% (0.04 M Mg2+) to 80% (0.18 M Mg2+) caused by the co-precipitation of Mg(OH)2(s) and MgCO3. Purities can be enhanced up to 100% by applying ethanol washing. It is worth noting that, when the basification step (in which Mg(OH)2 precipitates) is not followed by filtration (case at Mg2+ 0.08 M), a similar recovery of ∼75% is observed, while purity considerably drops from ∼87 to ∼68%. In this case, the ethanol washing step is not able to increase the purity above 90%, as it was also reported in Section 3.1.3. Such a result demonstrates that Mg(OH)2 precipitation and filtration before CO2 insufflation and Li2CO3 precipitation can be employed as a promising approach to first eliminate Mg2+ content in LiCl solutions and then obtain Li2CO3 solids with high purity (∼90%) and recovery (∼70%). 3.3. Comparison between Li+ Precipitation Using Na2CO3 and NaOH/CO2 Insufflation. The precipitation of Li2CO3 in LiCl solutions using either Na2CO3 or NaOH solutions and CO2(g) insufflation alternatives was extensively addressed in Sections 3.1 and 3.2. Table 1 reports a comparison between recovery and purity results obtained from the two precipitation approaches: (i) in the case of a double excess of the precipitants at 80 °C (reference case, see Sections 3.1.1 and 3.2.1), (ii) at a low temperature of 50 °C (see Figures 5a and 15a), (iii) in the presence of high-ionic strength solutions at 80 °C (Figures 5d and 15d), and (iv) in LiCl solution in the presence of 0.04 M Mg2+ concentration at 80 °C (Figures 9 and 17). From Table 1, it can be observed that temperature is a crucial parameter for Li recovery. The lowest recovery values of ∼50% are, in fact, achieved at 50 °C. Li recovery can be Table 1. Comparison between Li+ Recovery and Purity Obtained Using Either Na2CO3 or NaOH Solution and CO2(g) Insufflation Precipitation Routes reference case low temperature high ionic strength 0.04 M Mg concentration precipitation method Na2CO3, (CO32−/Li+ = 1) NaOH & CO2(g), (OH−/Li+ = 2) Na2CO3 NaOH & CO2(g) Na2CO3 NaOH & CO2(g) Na2CO3 NaOH & CO2(g) after filtration T [°C] 80 ∼62 ∼95 80 ∼60 ∼99 50 ∼55 ∼94 50 ∼50 ∼97 80 ∼77 ∼80 80 ∼80 ∼90 80 ∼65 ∼65 80 ∼70 ∼90 13598 ∼45 ∼100 ∼53 ∼100 ∼60 ∼100 ∼50 ∼75 ∼60 ∼100 https://doi.org/10.1021/acs.iecr.2c01397 recovery purity equivalent recovery after EtOH purity after EtOH washing [%] [%] washing [%] [ %] Ind. Eng. Chem. Res. 2022, 61, 13589−13602PDF Image | Recovery of Lithium Carbonate from Dilute Li Rich Brine
PDF Search Title:
Recovery of Lithium Carbonate from Dilute Li Rich BrineOriginal File Name Searched:
acs.iecr.2c01397.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |