
PDF Publication Title:
Text from PDF Page: 012
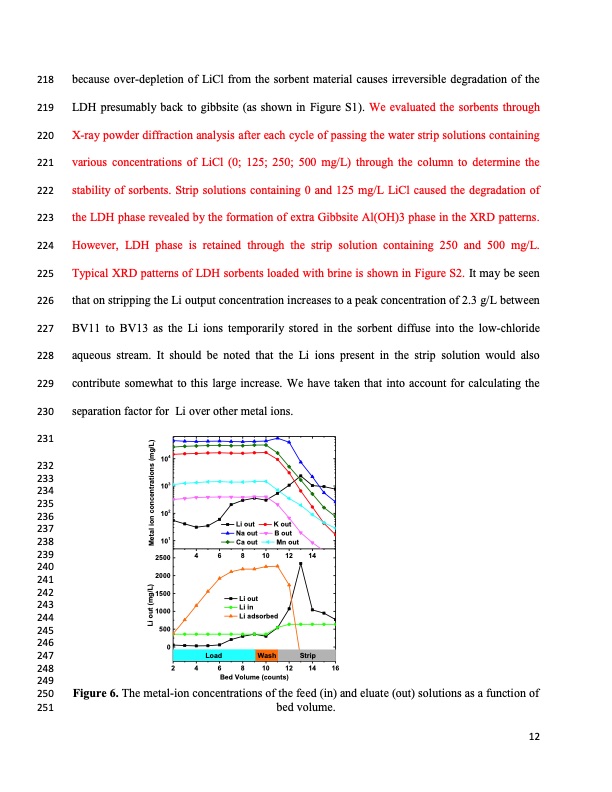
218 because over-depletion of LiCl from the sorbent material causes irreversible degradation of the 219 LDH presumably back to gibbsite (as shown in Figure S1). We evaluated the sorbents through 220 X-ray powder diffraction analysis after each cycle of passing the water strip solutions containing 221 various concentrations of LiCl (0; 125; 250; 500 mg/L) through the column to determine the 222 stability of sorbents. Strip solutions containing 0 and 125 mg/L LiCl caused the degradation of 223 the LDH phase revealed by the formation of extra Gibbsite Al(OH)3 phase in the XRD patterns. 224 However, LDH phase is retained through the strip solution containing 250 and 500 mg/L. 225 Typical XRD patterns of LDH sorbents loaded with brine is shown in Figure S2. It may be seen 226 that on stripping the Li output concentration increases to a peak concentration of 2.3 g/L between 227 BV11 to BV13 as the Li ions temporarily stored in the sorbent diffuse into the low-chloride 228 aqueous stream. It should be noted that the Li ions present in the strip solution would also 229 contribute somewhat to this large increase. We have taken that into account for calculating the 230 separation factor for Li over other metal ions. 231 Li out Na out Ca out K out B out Mn out 4 6 8 10 12 14 Li out Li in Li adsorbed Wash Load Strip 232 233 234 235 236 237 238 239 240 241 242 1500 243 104 103 102 101 244 245 246 247 2500 2000 1000 500 0 248 2 4 6 8 10 12 14 16 Bed Volume (counts) 249 250 Figure 6. The metal-ion concentrations of the feed (in) and eluate (out) solutions as a function of 251 bed volume. 12 Li out (mg/L) Metal ion concentrations (mg/L)PDF Image | Recovery of Lithium from Geothermal Brine Li AL
PDF Search Title:
Recovery of Lithium from Geothermal Brine Li ALOriginal File Name Searched:
1424451.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |