
PDF Publication Title:
Text from PDF Page: 005
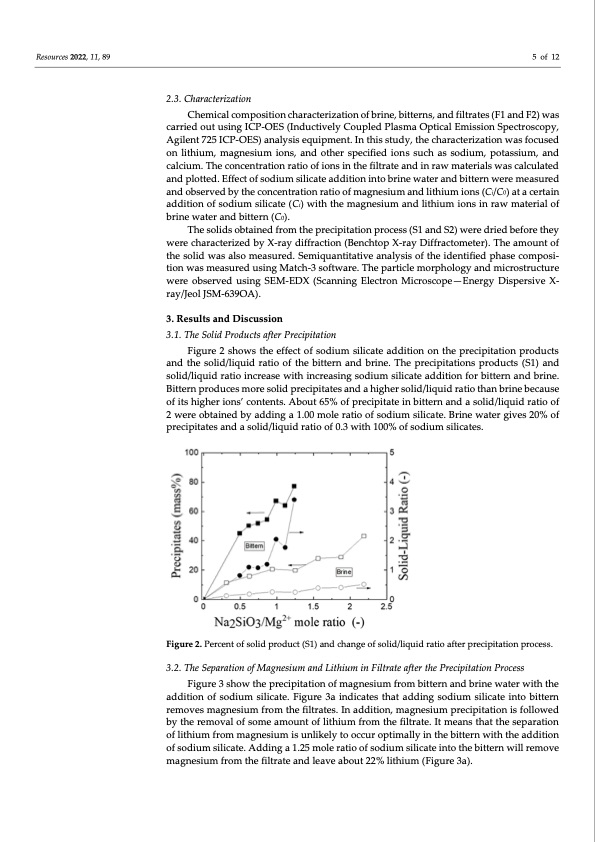
Resources 2022, 11, 89 5 of 12 2.3. Characterization Chemical composition characterization of brine, bitterns, and filtrates (F1 and F2) was carried out using ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy, Agilent 725 ICP-OES) analysis equipment. In this study, the characterization was focused on lithium, magnesium ions, and other specified ions such as sodium, potassium, and calcium. The concentration ratio of ions in the filtrate and in raw materials was calculated and plotted. Effect of sodium silicate addition into brine water and bittern were measured and observed by the concentration ratio of magnesium and lithium ions (Ci/C0) at a certain addition of sodium silicate (Ci) with the magnesium and lithium ions in raw material of brine water and bittern (C0). The solids obtained from the precipitation process (S1 and S2) were dried before they were characterized by X-ray diffraction (Benchtop X-ray Diffractometer). The amount of the solid was also measured. Semiquantitative analysis of the identified phase composi- tion was measured using Match-3 software. The particle morphology and microstructure were observed using SEM-EDX (Scanning Electron Microscope—Energy Dispersive X- ray/Jeol JSM-639OA). 3. Results and Discussion 3.1. The Solid Products after Precipitation Figure 2 shows the effect of sodium silicate addition on the precipitation products and the solid/liquid ratio of the bittern and brine. The precipitations products (S1) and solid/liquid ratio increase with increasing sodium silicate addition for bittern and brine. Bittern produces more solid precipitates and a higher solid/liquid ratio than brine because of its higher ions’ contents. About 65% of precipitate in bittern and a solid/liquid ratio of 2 were obtained by adding a 1.00 mole ratio of sodium silicate. Brine water gives 20% of precipitates and a solid/liquid ratio of 0.3 with 100% of sodium silicates. Figure 2. Percent of solid product (S1) and change of solid/liquid ratio after precipitation process. 3.2. The Separation of Magnesium and Lithium in Filtrate after the Precipitation Process Figure 3 show the precipitation of magnesium from bittern and brine water with the addition of sodium silicate. Figure 3a indicates that adding sodium silicate into bittern removes magnesium from the filtrates. In addition, magnesium precipitation is followed by the removal of some amount of lithium from the filtrate. It means that the separation of lithium from magnesium is unlikely to occur optimally in the bittern with the addition of sodium silicate. Adding a 1.25 mole ratio of sodium silicate into the bittern will remove magnesium from the filtrate and leave about 22% lithium (Figure 3a).PDF Image | Separation of Magnesium and Lithium from Brine Water
PDF Search Title:
Separation of Magnesium and Lithium from Brine WaterOriginal File Name Searched:
resources-11-00089.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |