
PDF Publication Title:
Text from PDF Page: 006
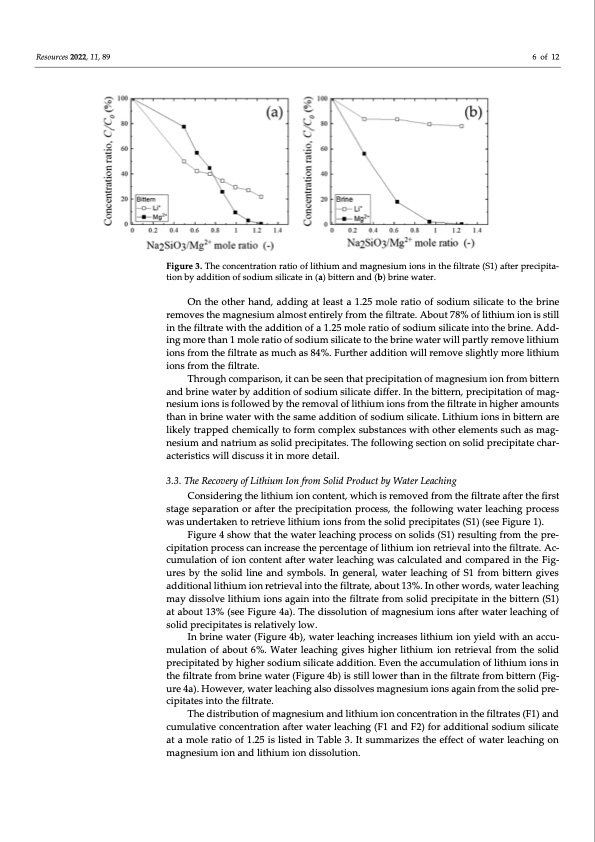
Resources 2022, 11, 89 6 of 12 Figure 3. The concentration ratio of lithium and magnesium ions in the filtrate (S1) after precipita- tion by addition of sodium silicate in (a) bittern and (b) brine water. On the other hand, adding at least a 1.25 mole ratio of sodium silicate to the brine removes the magnesium almost entirely from the filtrate. About 78% of lithium ion is still in the filtrate with the addition of a 1.25 mole ratio of sodium silicate into the brine. Add- ing more than 1 mole ratio of sodium silicate to the brine water will partly remove lithium ions from the filtrate as much as 84%. Further addition will remove slightly more lithium ions from the filtrate. Through comparison, it can be seen that precipitation of magnesium ion from bittern and brine water by addition of sodium silicate differ. In the bittern, precipitation of mag- nesium ions is followed by the removal of lithium ions from the filtrate in higher amounts than in brine water with the same addition of sodium silicate. Lithium ions in bittern are likely trapped chemically to form complex substances with other elements such as mag- nesium and natrium as solid precipitates. The following section on solid precipitate char- acteristics will discuss it in more detail. 3.3. The Recovery of Lithium Ion from Solid Product by Water Leaching Considering the lithium ion content, which is removed from the filtrate after the first stage separation or after the precipitation process, the following water leaching process was undertaken to retrieve lithium ions from the solid precipitates (S1) (see Figure 1). Figure 4 show that the water leaching process on solids (S1) resulting from the pre- cipitation process can increase the percentage of lithium ion retrieval into the filtrate. Ac- cumulation of ion content after water leaching was calculated and compared in the Fig- ures by the solid line and symbols. In general, water leaching of S1 from bittern gives additional lithium ion retrieval into the filtrate, about 13%. In other words, water leaching may dissolve lithium ions again into the filtrate from solid precipitate in the bittern (S1) at about 13% (see Figure 4a). The dissolution of magnesium ions after water leaching of solid precipitates is relatively low. In brine water (Figure 4b), water leaching increases lithium ion yield with an accu- mulation of about 6%. Water leaching gives higher lithium ion retrieval from the solid precipitated by higher sodium silicate addition. Even the accumulation of lithium ions in the filtrate from brine water (Figure 4b) is still lower than in the filtrate from bittern (Fig- ure 4a). However, water leaching also dissolves magnesium ions again from the solid pre- cipitates into the filtrate. The distribution of magnesium and lithium ion concentration in the filtrates (F1) and cumulative concentration after water leaching (F1 and F2) for additional sodium silicate at a mole ratio of 1.25 is listed in Table 3. It summarizes the effect of water leaching on magnesium ion and lithium ion dissolution.PDF Image | Separation of Magnesium and Lithium from Brine Water
PDF Search Title:
Separation of Magnesium and Lithium from Brine WaterOriginal File Name Searched:
resources-11-00089.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |