
PDF Publication Title:
Text from PDF Page: 010
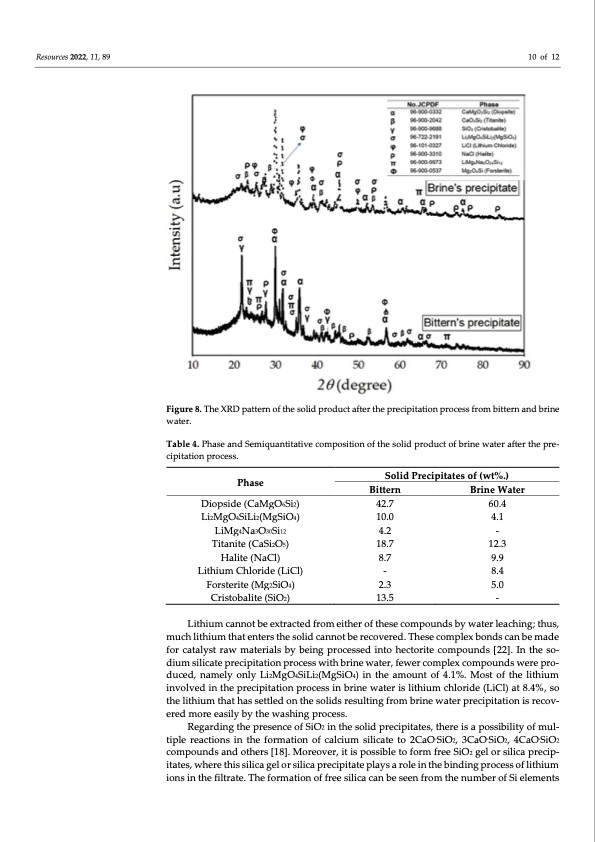
Resources 2022, 11, 89 10 of 12 Figure 8. The XRD pattern of the solid product after the precipitation process from bittern and brine water. Table 4. Phase and Semiquantitative composition of the solid product of brine water after the pre- cipitation process. Phase Diopside (CaMgO6Si2) Li2MgO4SiLi2(MgSiO4) LiMg4Na3O30Si12 Titanite (CaSi2O5) Halite (NaCl) Lithium Chloride (LiCl) Forsterite (Mg2SiO4) Cristobalite (SiO2) Solid Precipitates of (wt%.) Bittern Brine Water 42.7 60.4 10.0 4.1 4.2 - 18.7 12.3 8.7 9.9 - 8.4 2.3 5.0 13.5 - Lithium cannot be extracted from either of these compounds by water leaching; thus, much lithium that enters the solid cannot be recovered. These complex bonds can be made for catalyst raw materials by being processed into hectorite compounds [22]. In the so- dium silicate precipitation process with brine water, fewer complex compounds were pro- duced, namely only Li2MgO4SiLi2(MgSiO4) in the amount of 4.1%. Most of the lithium involved in the precipitation process in brine water is lithium chloride (LiCl) at 8.4%, so the lithium that has settled on the solids resulting from brine water precipitation is recov- ered more easily by the washing process. Regarding the presence of SiO2 in the solid precipitates, there is a possibility of mul- tiple reactions in the formation of calcium silicate to 2CaO.SiO2, 3CaO.SiO2, 4CaO.SiO2 compounds and others [18]. Moreover, it is possible to form free SiO2 gel or silica precip- itates, where this silica gel or silica precipitate plays a role in the binding process of lithium ions in the filtrate. The formation of free silica can be seen from the number of Si elementsPDF Image | Separation of Magnesium and Lithium from Brine Water
PDF Search Title:
Separation of Magnesium and Lithium from Brine WaterOriginal File Name Searched:
resources-11-00089.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |