
PDF Publication Title:
Text from PDF Page: 005
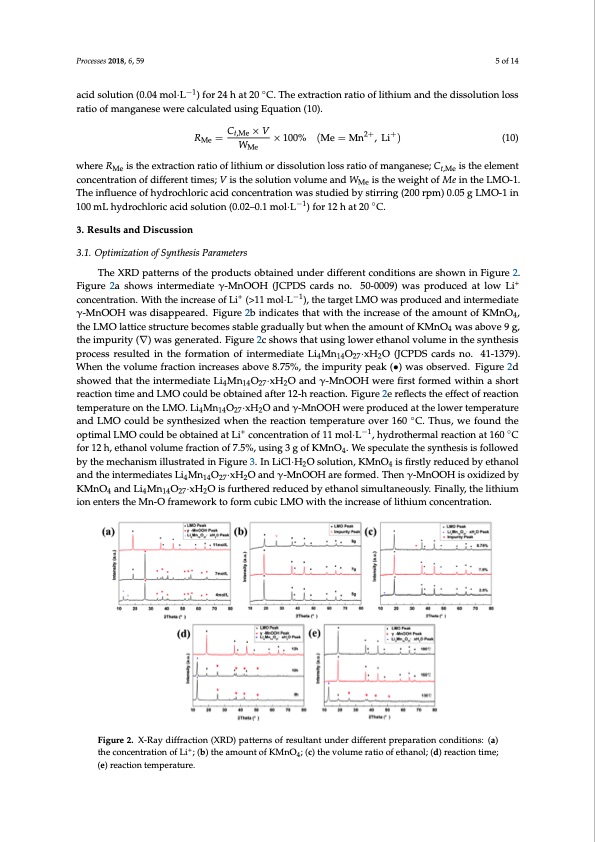
Processes 2018, 6, 59 5 of 14 acid solution (0.04 mol·L−1) for 24 h at 20 ◦C. The extraction ratio of lithium and the dissolution loss ratio of manganese were calculated using Equation (10). RMe = Ct,Me × V × 100% (Me = Mn2+, Li+) (10) WMe where RMe is the extraction ratio of lithium or dissolution loss ratio of manganese; Ct,Me is the element Processes 2018, 6, x FOR PEER REVIEW 5 of 14 concentration of different times; V is the solution volume and WMe is the weight of Me in the LMO-1. The influence of hydrochloric acid concentration was studied by stirring (200 rpm) 0.05 g LMO-1 in where is the extraction ratio of lithium or dissolution loss ratio of manganese; , is the 100 mL hydrochloric acid solution (0.02–0.1 mol·L−1) for 12 h at 20 ◦C. element concentration of different times; V is the solution volume and is the weight of in the LMO-1. The influence of hydrochloric acid concentration was studied by stirring (200 rpm) 0.05 g 3. Results and Discussion LMO-1 in 100 mL hydrochloric acid solution (0.02–0.1 mol·L−1) for 12 h at 20 °C. 3.1. Optimization of Synthesis Parameters 3. Results and Discussion The XRD patterns of the products obtained under different conditions are shown in Figure 2. 3.1. Optimization of Synthesis Parameters + Figure 2a shows intermediate γ-MnOOH (JCPDS cards no. 50-0009) was produced at low Li The XRD patterns of the prod+ucts obtained−u1nder different conditions are shown in Figure 2. concentration. With the increase of Li (>11 mol·L ), the target LMO was produced and intermediate Figure 2a shows intermediate γ-MnOOH (JCPDS cards no. 50-0009) was produced at low Li+ γ-MnOOH was disappeared. Figure 2b indicates that with the increase of the amount of KMnO4, concentration. With the increase of Li+ (>11 mol·L−1), the target LMO was produced and intermediate the LMO lattice structure becomes stable gradually but when the amount of KMnO4 was above 9 g, γ-MnOOH was disappeared. Figure 2b indicates that with the increase of the amount of KMnO4, the the impurity (∇) was generated. Figure 2c shows that using lower ethanol volume in the synthesis LMO lattice structure becomes stable gradually but when the amount of KMnO4 was above 9 g, the process resulted in the formation of intermediate Li Mn O ·xH O (JCPDS cards no. 41-1379). 4 14 27 2 impurity (∇) was generated. Figure 2c shows that using lower ethanol volume in the synthesis When the volume fraction increases above 8.75%, the impurity peak (•) was observed. Figure 2d process resulted in the formation of intermediate Li4Mn14O27·xH2O (JCPDS cards no. 41-1379). When showed that the intermediate Li Mn O ·xH O and γ-MnOOH were first formed within a short the volume fraction increases a4bove184.752%7 , the2 impurity peak (•) was observed. Figure 2d showed reactitohnatttihmeeinatnerdmLedMiaOtecLoi4uMldn1b4Oe2o7·bxHta2iOneadndafγt-eMr n1O2-OhHrewacetrieofnir.sFt fiogrumred2ewritehflinecatshtohret reefafectcitonoftirmeaection and LMO could be obtained after 12-h reaction. Figure 2e reflects the effect of reaction temperature temperature on the LMO. Li4Mn14O27·xH2O and γ-MnOOH were produced at the lower temperature on the LMO. Li4Mn14O27·xH2O and γ-MnOOH were produced at the lower te◦mperature and LMO and LMO could be synthesized when the reaction temperature over 160 C. Thus, we found the could be synthesized when the reac+tion temperature over 160 °C. T−h1us, we found the optimal LMO ◦ optimal LMO could be obtained at Li concentration of 11 mol·L , hydrothermal reaction at 160 could be obtained at Li+ concentration of 11 mol·L−1, hydrothermal reaction at 160 °C for 12 h, C for 12 h, ethanol volume fraction of 7.5%, using 3 g of KMnO4. We speculate the synthesis is followed ethanol volume fraction of 7.5%, using 3 g of KMnO4. We speculate the synthesis is followed by the by the mechanism illustrated in Figure 3. In LiCl·H2O solution, KMnO4 is firstly reduced by ethanol mechanism illustrated in Figure 3. In LiCl·H2O solution, KMnO4 is firstly reduced by ethanol and the and the intermediates Li4Mn14O27·xH2O and γ-MnOOH are formed. Then γ-MnOOH is oxidized by intermediates Li4Mn14O27·xH2O and γ-MnOOH are formed. Then γ-MnOOH is oxidized by KMnO4 KMnO and Li Mn O ·xH O is furthered reduced by ethanol simultaneously. Finally, the lithium an4d Li4Mn14O27·x1H4 2O27is fur2thered reduced by ethanol simultaneously. Finally, the lithium ion enters ion enthterMs nth-Oe Mfranm-Oewforarkmteowforrmk ctuobfiocrLmMcOubwiicthLtMheOinwcrietahsethoef liinthcriuemasecoonfcleinthtriautmionc.oncentration. Figure 2. X-Ray diffraction (XRD) patterns of resultant under different preparation Figure 2. X-Ray diffraction (XRD) patterns of resultant under different preparation conditions: (a) conditions: (a) the concentration of Li+; (b) the amount of KMnO4; (c) the volume ratio of the concentration of Li+; (b) the amount of KMnO4; (c) the volume ratio of ethanol; (d) reaction time; ethanol; (d) reaction time; (e) reaction temperature. (e) reaction temperature.PDF Image | Sieves for Highly Selective Li Adsorption
PDF Search Title:
Sieves for Highly Selective Li AdsorptionOriginal File Name Searched:
processes-06-00059-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |