
PDF Publication Title:
Text from PDF Page: 011
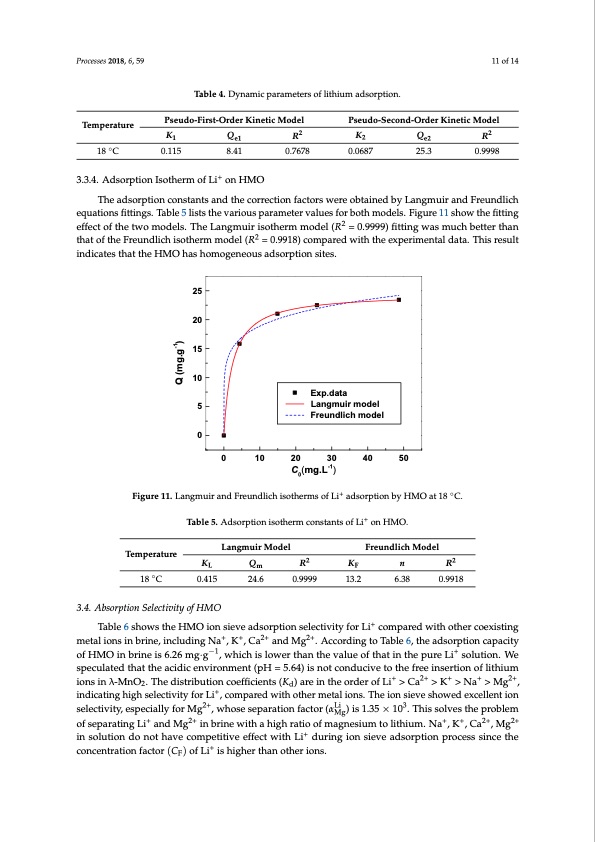
Temperature ◦ Table 4. Dynamic parameters of lithium adsorption. PseudoT-Faibrslet-O4.rDdyernaKminicetpicarMamodeteelrs of lithiumPsaedusdoorp-Stieocno.nd-Order Kinetic Model -1.0 -1.5 -2.0 0 5 10 15 20 Time (h) 25 30 0.5 0.0 -0.5 Processes 2018, 6, 59 Figure 10. Pseudo-first-order and pseudo-second-order kinetic curves Li+ adsorption by HMO at 18 °C. 11 of 14 22 K Pseudo-FiQrst-Order Kinetic MRodel Pseudo-KSecond-Order KiQnetic Model R 18 °C 0.115 8.41 3.3.4. Adsorption Isotherm of Li+ on HMO 0.7678 0.0687 25.3 1e1 2e2 25.3 0.9998 Temperature 0.115 8.41 0.9998 18 C 3.3.4. Adsorption Isotherm of Li+ on HMO 0.7678 0.0687 The adsorption constants and the correction factors were obtained by Langmuir and Freundlich The adsorption constants and the correction factors were obtained by Langmuir and Freundlich equations fittings. Table 5 lists the various parameter values for both models. Figure 11 show the fitting equations fittings. Table 5 lists the various parameter values for both models. Figure 11 show the effect of the two models. The Langmuir isotherm model (R2 = 0.9999) fitting was much better than fitting effect of the two models. The Langmuir isotherm model ( = 0.9999) fitting was much better that of the Freundlich isotherm model (R2 = 0.9918) compared with the experimental data. This result than that of the Freundlich isotherm model ( = 0.9918) compared with the experimental data. This indicates that the HMO has homogeneous adsorption sites. result indicates that the HMO has homogeneous adsorption sites. Exp.data Langmuir model Freundlich model 25 20 15 10 5 0 0 10 20 30 40 50 C0(mg.L-1) Figure 11. Langmuir and Freundlich isotherms of L+i+ adsorption by HMO at◦18 °C. Figure 11. Langmuir and Freundlich isotherms of Li adsorption by HMO at 18 C. + Table 5. Adsorption isotherm constants of Li+ on HMO. Table 5. Adsorption isotherm constants of Li on HMO. Temperature Langmuir Model Freundlich Model Table 6 shows the HMO ion sieve adsorption selectivity for Li+ compared with other coexisting metal ions in brine, including Na+, K+, Ca2+ and Mg2+. According to Table 6, the adsorption capacity of HMO in brine is 6.26 mg·g−1, which is lower than the value of that in the pure Li+ solution. We speculated that the acidic environment (pH = 5.64) is not conducive to the free insertion of lithium ions in λ-MnO2. The distribution coefficients (Kd) are in the order of Li+ > Ca2+ > K+ > Na+ > Mg2+, indicating high selectivity for Li+, compared with other metal ions. The ion sieve showed excellent ion selectivity,especiallyforMg2+,whoseseparationfactor(αLi )is1.35×103.Thissolvestheproblem Mg of separating Li+ and Mg2+ in brine with a high ratio of magnesium to lithium. Na+, K+, Ca2+, Mg2+ in solution do not have competitive effect with Li+ during ion sieve adsorption process since the concentration factor (CF) of Li+ is higher than other ions. Temperature Langmuir Model Freundlich Model 18 °C 0.415 24.6 0.9999 13.2 6.38 0.9918 KL Qm R2 KF n R2 0.415 24.6 0.9999 13.2 6.38 0.9918 18 ◦ C 3.4. Absorption Selectivity of HMO Q (mg.g-1) lg(PDF Image | Sieves for Highly Selective Li Adsorption
PDF Search Title:
Sieves for Highly Selective Li AdsorptionOriginal File Name Searched:
processes-06-00059-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |