
PDF Publication Title:
Text from PDF Page: 012
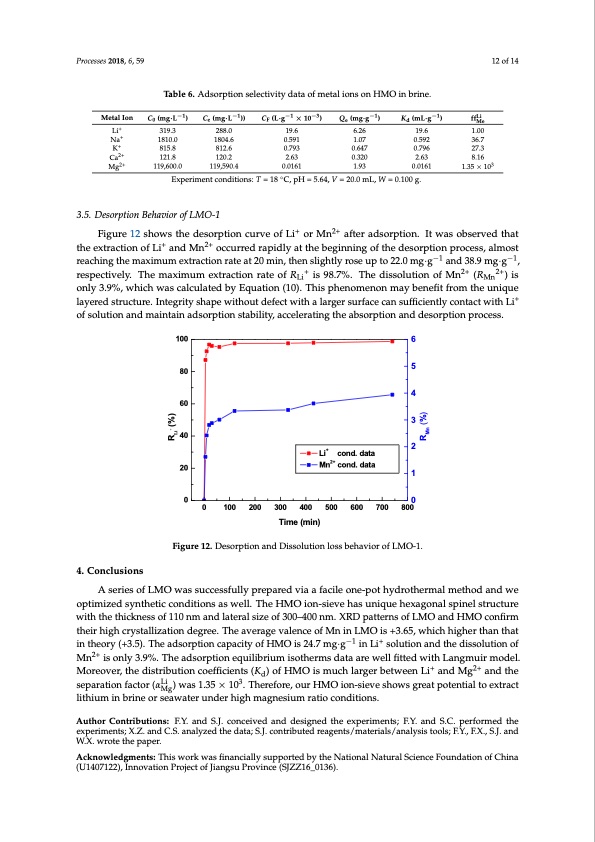
ions in λ-MnO2. The distribution coefficients ( ) are in the order of Li+ > Ca2+ > K+ > Na+ > Mg2+, indicating high selectivity for Li+, compared with other metal ions. The ion sieve showed excellent ion selectivity, especially for Mg2+, whose separation factor ( ) is 1.35 × 103. This solves the problem of separating Li+ and Mg2+ in brine with a high ratio of magnesium to lithium. Na+, K+, Ca2+, Mg2+ in solution do not have competitive effect with Li+ during ion sieve adsorption process since the Processes 2018, 6, 59 concentration factor of Li+ is higher than other ions. Table 6. Adsorption selectivity data of metal ions on HMO in briine.. −1 −1 −1−3 −1 −1 MetalIon (m−g1·L ) (m−g1·L )) −(L1·g ×−130 ) (m−g1·g ) (mL−·g1 ) Li MetalIon C0(mg·L ) Ce(mg·L )) CF(L·g ×10 ) Qe(mg·g ) Kd(mL·g ) ff 12 of 14 +Li+ Li ++ NaNa 319.3 288.0 19.6 6.26 19.6 Me K+ + K 0.5.59292 1.00 3366..7 Ca2+ 815.8 812.6 0.793 0.647 0.796 27.3 C2+a2+ Mg 121.8 120.2 2.63 120.2 2.63 0.320 2.63 8.16 8.16 3 Mg2+ 119,600.0 119,590.4 ◦ 0.0161 C, pH = 5.64, V = 20.0 mL, W = 0.100 g. 319.3 18108.010.0 288.0 19.6 18014.8604.6 0.5091.591 6.26 1.017.07 19.6 1.00 815.8 812.6 0.793 0.647 0.796 27.3 119,600.0 119,590.4 0.0161 1.93 0.0161 121.8 0.320 2.63 1.35 × 10 1.35×103 1.93 Experiment conditions: T = 18 °C, pH = 5.64, V = 20.0 mL, W = 0.100 g. Experiment conditions: T = 18 0.0161 3.5. Desorption Behavior of LMO-1 Figure 12 showsthedesorrpttiionccurrvveeooffLLiiororMMnnaftaefrteardasodrspotripotni.oInt.wItaswoabssoerbvsedrvtehdatththaet ++ 2+2+ tehxetreaxctrioanctiofn Loif LaindanMd nMnoccoucrcreudrrerdapriadplyidlayt atthtehebebgeignininigngofofththee desorrpttiion process, almost ++ 2+2+ reachingtthemaxiimumeexxttrraacctitoionnrarateteaatt2200mminin, t,hthenenslsiglihgthlytlyrorsoeseupuptoto222.02.m0 mg·g·g aanndd3388.9.9mgg·g·g , ++ 2+2+2+ 2+ respectively. ThemaxiimumeexxttrraacctitoionnrraateteooffRRLi isi9s89.78%.7.%T.hTehdeisdsoislsuotilountionf MofnMn(RMn(R) is o)nliys Li Mn o3.n9l%y ,3w.9h%ic, hwwhiacshcwalcauslcaatelcdublayteEdqubaytiEoqnu(a1t0i)o.nTh(1i0s)p. hTehniosmphenenonomeanyobnemneafyit bfreonmefitthefruomniqtuhe luanyieqruede ++ lsatyruercetudrset.ruIncteugreri.tIyntsehgarpiteywshiathpoeuwtidtheofeucttdwefiethctawliathrgaerlasrguerrfascuerfcacnecsaunffiscuieffinctileynctloynctaocnttawctitwhitLhiLoif osoflsuotliuotnioanadnmdaminatianitnaiandasdorspotripotniosntasbtailbitiyli,tya,cacceclerlaertiantigntghtehaebasbosroprtpiotnioannadndedseosroprtpiotinonprporcoecsess.s. 100 6 4. Conclusions A series of LMO was successfully prepared via a facile one-pot hydrothermal method and we A series of LMO was successfully prepared via a facile one-pot hydrothermal method and we optimized synthetic conditions as well. The HMO ion-sieve has unique hexagonal spinel structure optimized synthetic conditions as well. The HMO ion-sieve has unique hexagonal spinel structure with the thickness of 110 nm and lateral size of 300–400 nm. XRD patterns of LMO and HMO with the thickness of 110 nm and lateral size of 300–400 nm. XRD patterns of LMO and HMO confirm confirm their high crystallization degree. The average valence of Mn in LMO is +3.65, which higher their high crystallization degree. The average valence of Mn in LMO is +3.65, which higher than that in theory (+3.5). The adsorption capacity of HMO is 24.7 mg·g−1 in Li+ solution and the dissolution of Mn2+ is only 3.9%. The adsorption equilibrium isotherms data are well fitted with Langmuir model. Moreover, the distribution coefficients (Kd) of HMO is much larger between Li+ and Mg2+ and the separationfactor(αLi )was1.35×103.Therefore,ourHMOion-sieveshowsgreatpotentialtoextract Mg lithium in brine or seawater under high magnesium ratio conditions. Author Contributions: F.Y. and S.J. conceived and designed the experiments; F.Y. and S.C. performed the experiments; X.Z. and C.S. analyzed the data; S.J. contributed reagents/materials/analysis tools; F.Y., F.X., S.J. and W.X. wrote the paper. Acknowledgments: This work was financially supported by the National Natural Science Foundation of China (U1407122), Innovation Project of Jiangsu Province (SJZZ16_0136). −1−1 −−1 Li+ cond. data Mn2+ cond. data 4. Conclusions 80 60 40 20 5 4 3 2 1 00 0 100 200 300 400 500 600 700 800 Time (min) Figure 12. Desorption and Dissolution loss behavior of LMO-1. Figure 12. Desorption and Dissolution loss behavior of LMO-1. R + (%) Li RMn (%)PDF Image | Sieves for Highly Selective Li Adsorption
PDF Search Title:
Sieves for Highly Selective Li AdsorptionOriginal File Name Searched:
processes-06-00059-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |