
PDF Publication Title:
Text from PDF Page: 007
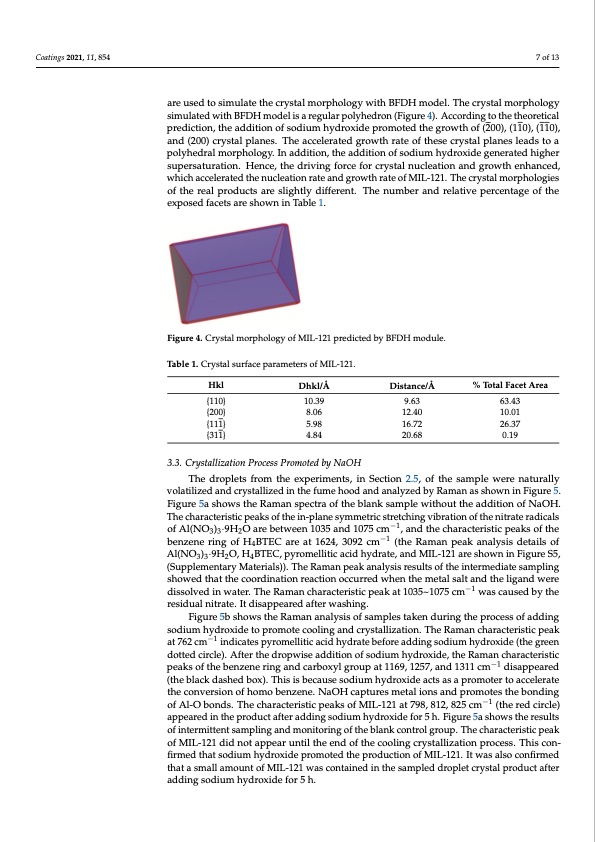
Coatings 2021, 11, 854 7 of 13 are used to simulate the crystal morphology with BFDH model. The crystal morphology simulated with BFDH model is a regular polyhedron (Figure 4). According to the theoretical prediction, the addition of sodium hydroxide promoted the growth of (200), (110), (110), and (200) crystal planes. The accelerated growth rate of these crystal planes leads to a polyhedral morphology. In addition, the addition of sodium hydroxide generated higher supersaturation. Hence, the driving force for crystal nucleation and growth enhanced, which accelerated the nucleation rate and growth rate of MIL-121. The crystal morphologies of the real products are slightly different. The number and relative percentage of the exposed facets are shown in Table 1. Figure 4. Crystal morphology of MIL-121 predicted by BFDH module. Table 1. Crystal surface parameters of MIL-121. Hkl {110} {200} {111} {311} Dhkl/Å Distance/Å 10.39 9.63 8.06 12.40 5.98 16.72 4.84 20.68 % Total Facet Area 63.43 10.01 26.37 0.19 3.3. Crystallization Process Promoted by NaOH The droplets from the experiments, in Section 2.5, of the sample were naturally volatilized and crystallized in the fume hood and analyzed by Raman as shown in Figure 5. Figure 5a shows the Raman spectra of the blank sample without the addition of NaOH. The characteristic peaks of the in-plane symmetric stretching vibration of the nitrate radicals of Al(NO3)3·9H2O are between 1035 and 1075 cm−1, and the characteristic peaks of the benzene ring of H4BTEC are at 1624, 3092 cm−1 (the Raman peak analysis details of Al(NO3)3·9H2O, H4BTEC, pyromellitic acid hydrate, and MIL-121 are shown in Figure S5, (Supplementary Materials)). The Raman peak analysis results of the intermediate sampling showed that the coordination reaction occurred when the metal salt and the ligand were dissolved in water. The Raman characteristic peak at 1035~1075 cm−1 was caused by the residual nitrate. It disappeared after washing. Figure 5b shows the Raman analysis of samples taken during the process of adding sodium hydroxide to promote cooling and crystallization. The Raman characteristic peak at 762 cm−1 indicates pyromellitic acid hydrate before adding sodium hydroxide (the green dotted circle). After the dropwise addition of sodium hydroxide, the Raman characteristic peaks of the benzene ring and carboxyl group at 1169, 1257, and 1311 cm−1 disappeared (the black dashed box). This is because sodium hydroxide acts as a promoter to accelerate the conversion of homo benzene. NaOH captures metal ions and promotes the bonding of Al-O bonds. The characteristic peaks of MIL-121 at 798, 812, 825 cm−1 (the red circle) appeared in the product after adding sodium hydroxide for 5 h. Figure 5a shows the results of intermittent sampling and monitoring of the blank control group. The characteristic peak of MIL-121 did not appear until the end of the cooling crystallization process. This con- firmed that sodium hydroxide promoted the production of MIL-121. It was also confirmed that a small amount of MIL-121 was contained in the sampled droplet crystal product after adding sodium hydroxide for 5 h.PDF Image | Small Particles for Lithium Adsorption from Brine
PDF Search Title:
Small Particles for Lithium Adsorption from BrineOriginal File Name Searched:
coatings-11-00854-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |