
PDF Publication Title:
Text from PDF Page: 003
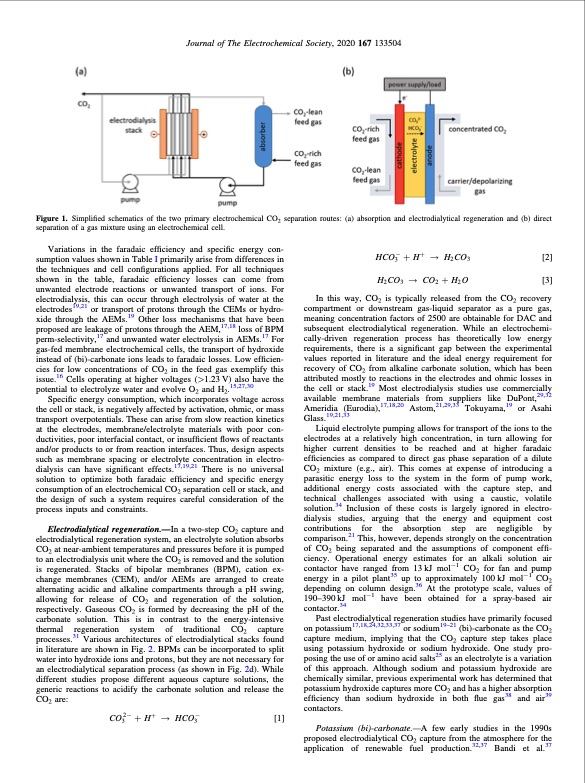
Journal of The Electrochemical Society, 2020 167 133504 Figure 1. Simplified schematics of the two primary electrochemical CO2 separation routes: (a) absorption and electrodialytical regeneration and (b) direct separation of a gas mixture using an electrochemical cell. Variations in the faradaic efficiency and specific energy con- sumption values shown in Table I primarily arise from differences in the techniques and cell configurations applied. For all techniques shown in the table, faradaic efficiency losses can come from unwanted electrode reactions or unwanted transport of ions. For electrodialysis, this can occur through electrolysis of water at the electrodes19,21 or transport of protons through the CEMs or hydro- xide through the AEMs.19 Other loss mechanisms that have been proposed are leakage of protons through the AEM,17,18 loss of BPM perm-selectivity,17 and unwanted water electrolysis in AEMs.17 For gas-fed membrane electrochemical cells, the transport of hydroxide instead of (bi)-carbonate ions leads to faradaic losses. Low efficien- cies for low concentrations of CO2 in the feed gas exemplify this issue.16 Cells operating at higher voltages (>1.23 V) also have the potential to electrolyze water and evolve O2 and H2.15,27,30 Specific energy consumption, which incorporates voltage across the cell or stack, is negatively affected by activation, ohmic, or mass transport overpotentials. These can arise from slow reaction kinetics at the electrodes, membrane/electrolyte materials with poor con- ductivities, poor interfacial contact, or insufficient flows of reactants and/or products to or from reaction interfaces. Thus, design aspects such as membrane spacing or electrolyte concentration in electro- dialysis can have significant effects.17,19,21 There is no universal solution to optimize both faradaic efficiency and specific energy consumption of an electrochemical CO2 separation cell or stack, and the design of such a system requires careful consideration of the process inputs and constraints. Electrodialytical regeneration.—In a two-step CO2 capture and electrodialytical regeneration system, an electrolyte solution absorbs CO2 at near-ambient temperatures and pressures before it is pumped to an electrodialysis unit where the CO2 is removed and the solution is regenerated. Stacks of bipolar membranes (BPM), cation ex- change membranes (CEM), and/or AEMs are arranged to create alternating acidic and alkaline compartments through a pH swing, allowing for release of CO2 and regeneration of the solution, respectively. Gaseous CO2 is formed by decreasing the pH of the carbonate solution. This is in contrast to the energy-intensive thermal regeneration system of traditional CO2 capture processes.31 Various architectures of electrodialytical stacks found in literature are shown in Fig. 2. BPMs can be incorporated to split water into hydroxide ions and protons, but they are not necessary for an electrodialytical separation process (as shown in Fig. 2d). While different studies propose different aqueous capture solutions, the generic reactions to acidify the carbonate solution and release the CO2 are: CO2- + H+ HCO- [1] 33 HCO- + H+ H CO [2] 323 H2CO3 CO2+H2O [3] In this way, CO2 is typically released from the CO2 recovery compartment or downstream gas-liquid separator as a pure gas, meaning concentration factors of 2500 are obtainable for DAC and subsequent electrodialytical regeneration. While an electrochemi- cally-driven regeneration process has theoretically low energy requirements, there is a significant gap between the experimental values reported in literature and the ideal energy requirement for recovery of CO2 from alkaline carbonate solution, which has been attributed mostly to reactions in the electrodes and ohmic losses in the cell or stack.19 Most electrodialysis studies use commercially available membrane materials from suppliers like DuPont,29,32 Ameridia (Eurodia),17,18,20 Astom,21,29,33 Tokuyama,19 or Asahi Glass.19,21,33 Liquid electrolyte pumping allows for transport of the ions to the electrodes at a relatively high concentration, in turn allowing for higher current densities to be reached and at higher faradaic efficiencies as compared to direct gas phase separation of a dilute CO2 mixture (e.g., air). This comes at expense of introducing a parasitic energy loss to the system in the form of pump work, additional energy costs associated with the capture step, and technical challenges associated with using a caustic, volatile solution.34 Inclusion of these costs is largely ignored in electro- dialysis studies, arguing that the energy and equipment cost contributions for the absorption step are negligible by comparison.21 This, however, depends strongly on the concentration of CO2 being separated and the assumptions of component effi- ciency. Operational energy estimates for an alkali solution air contactor have ranged from 13 kJ mol−1 CO2 for fan and pump energy in a pilot plant35 up to approximately 100 kJ mol−1 CO2 depending on column design.36 At the prototype scale, values of 190–390kJ mol−1 have been obtained for a spray-based air contactor.34 Past electrodialytical regeneration studies have primarily focused on potassium17,18,24,32,33,37 or sodium19–21 (bi)-carbonate as the CO2 capture medium, implying that the CO2 capture step takes place using potassium hydroxide or sodium hydroxide. One study pro- posing the use of or amino acid salts25 as an electrolyte is a variation of this approach. Although sodium and potassium hydroxide are chemically similar, previous experimental work has determined that potassium hydroxide captures more CO2 and has a higher absorption efficiency than sodium hydroxide in both flue gas38 and air39 contactors. Potassium (bi)-carbonate.—A few early studies in the 1990s proposed electrodialytical CO2 capture from the atmosphere for the application of renewable fuel production.32,37 Bandi et al.37PDF Image | CO2 Separation and Transport via Electrochemical Methods
PDF Search Title:
CO2 Separation and Transport via Electrochemical MethodsOriginal File Name Searched:
co2-separation-electrochemical.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |