
PDF Publication Title:
Text from PDF Page: 003
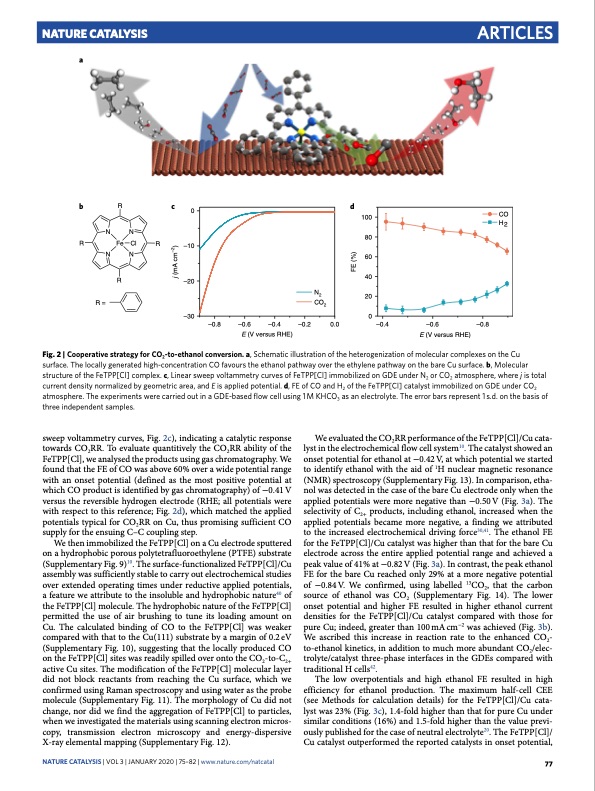
Nature Catalysis a Articles bRc0d 100 CO H2 NN R FeClR NN –10 80 60 40 20 0 j (mA cm–2) FE (%) R –20 R= CO2 –30 –0.8 –0.6 –0.4 –0.2 E (V versus RHE) Fig. 2 | Cooperative strategy for CO2-to-ethanol conversion. a, Schematic illustration of the heterogenization of molecular complexes on the Cu surface. The locally generated high-concentration CO favours the ethanol pathway over the ethylene pathway on the bare Cu surface. b, Molecular structure of the FeTPP[Cl] complex. c, Linear sweep voltammetry curves of FeTPP[Cl] immobilized on GDE under n2 or CO2 atmosphere, where j is total current density normalized by geometric area, and E is applied potential. d, FE of CO and H2 of the FeTPP[Cl] catalyst immobilized on GDE under CO2 atmosphere. The experiments were carried out in a GDE-based flow cell using 1 M KHCO3 as an electrolyte. The error bars represent 1 s.d. on the basis of three independent samples. sweep voltammetry curves, Fig. 2c), indicating a catalytic response towards CO2RR. To evaluate quantitively the CO2RR ability of the FeTPP[Cl], we analysed the products using gas chromatography. We found that the FE of CO was above 60% over a wide potential range with an onset potential (defined as the most positive potential at which CO product is identified by gas chromatography) of −0.41 V versus the reversible hydrogen electrode (RHE; all potentials were with respect to this reference; Fig. 2d), which matched the applied potentials typical for CO2RR on Cu, thus promising sufficient CO supply for the ensuing C–C coupling step. We then immobilized the FeTPP[Cl] on a Cu electrode sputtered on a hydrophobic porous polytetrafluoroethylene (PTFE) substrate (Supplementary Fig. 9)10. The surface-functionalized FeTPP[Cl]/Cu assembly was sufficiently stable to carry out electrochemical studies over extended operating times under reductive applied potentials, a feature we attribute to the insoluble and hydrophobic nature40 of the FeTPP[Cl] molecule. The hydrophobic nature of the FeTPP[Cl] permitted the use of air brushing to tune its loading amount on Cu. The calculated binding of CO to the FeTPP[Cl] was weaker compared with that to the Cu(111) substrate by a margin of 0.2 eV (Supplementary Fig. 10), suggesting that the locally produced CO on the FeTPP[Cl] sites was readily spilled over onto the CO2-to-C2+ active Cu sites. The modification of the FeTPP[Cl] molecular layer did not block reactants from reaching the Cu surface, which we confirmed using Raman spectroscopy and using water as the probe molecule (Supplementary Fig. 11). The morphology of Cu did not change, nor did we find the aggregation of FeTPP[Cl] to particles, when we investigated the materials using scanning electron micros- copy, transmission electron microscopy and energy-dispersive X-ray elemental mapping (Supplementary Fig. 12). We evaluated the CO2RR performance of the FeTPP[Cl]/Cu cata- lyst in the electrochemical flow cell system10. The catalyst showed an onset potential for ethanol at −0.42 V, at which potential we started to identify ethanol with the aid of 1H nuclear magnetic resonance (NMR) spectroscopy (Supplementary Fig. 13). In comparison, etha- nol was detected in the case of the bare Cu electrode only when the applied potentials were more negative than −0.50 V (Fig. 3a). The selectivity of C2+ products, including ethanol, increased when the applied potentials became more negative, a finding we attributed to the increased electrochemical driving force30,41. The ethanol FE for the FeTPP[Cl]/Cu catalyst was higher than that for the bare Cu electrode across the entire applied potential range and achieved a peak value of 41% at −0.82 V (Fig. 3a). In contrast, the peak ethanol FE for the bare Cu reached only 29% at a more negative potential of −0.84V. We confirmed, using labelled 13CO2, that the carbon source of ethanol was CO2 (Supplementary Fig. 14). The lower onset potential and higher FE resulted in higher ethanol current densities for the FeTPP[Cl]/Cu catalyst compared with those for pure Cu; indeed, greater than 100mAcm−2 was achieved (Fig. 3b). We ascribed this increase in reaction rate to the enhanced CO2- to-ethanol kinetics, in addition to much more abundant CO2/elec- trolyte/catalyst three-phase interfaces in the GDEs compared with traditional H cells42. The low overpotentials and high ethanol FE resulted in high efficiency for ethanol production. The maximum half-cell CEE (see Methods for calculation details) for the FeTPP[Cl]/Cu cata- lyst was 23% (Fig. 3c), 1.4-fold higher than that for pure Cu under similar conditions (16%) and 1.5-fold higher than the value previ- ously published for the case of neutral electrolyte20. The FeTPP[Cl]/ Cu catalyst outperformed the reported catalysts in onset potential, NATurE CATALYSiS | VOL 3 | JanUarY 2020 | 75–82 | www.nature.com/natcatal 77 N2 0.0 –0.4 –0.6 –0.8 E (V versus RHE)PDF Image | Cooperative CO2-to-ethanol conversion via enriched intermediates
PDF Search Title:
Cooperative CO2-to-ethanol conversion via enriched intermediatesOriginal File Name Searched:
s41929-019-0383-7.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |