
PDF Publication Title:
Text from PDF Page: 005
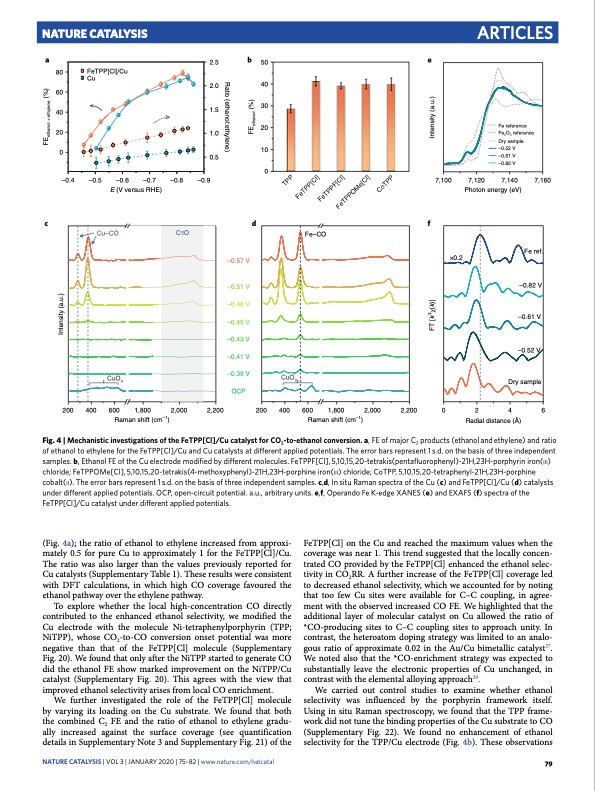
Nature Catalysis Articles a 2.5 2.0 1.5 1.0 0.5 b 50 e 40 30 20 10 0 80 60 40 20 0 FeTPP[Cl]/Cu Cu Ratio (ethanol:ethylene) –0.4 –0.5 –0.6 –0.7 –0.8 –0.9 E (V versus RHE) 7,100 7,120 Photon energy (eV) 7,160 cdf Cu–CO C O Fe–CO –0.57 V –0.51 V –0.48 V –0.45 V –0.43 V –0.41 V ×0.2 Fe ref. –0.82 V –0.61 V –0.52 V –0.39 V CuOx OCP CuO Raman shift (cm–1) Raman shift (cm–1) Radial distance (Å) Fig. 4 | Mechanistic investigations of the FeTPP[Cl]/Cu catalyst for CO2-to-ethanol conversion. a, FE of major C2 products (ethanol and ethylene) and ratio of ethanol to ethylene for the FeTPP[Cl]/Cu and Cu catalysts at different applied potentials. The error bars represent 1 s.d. on the basis of three independent samples. b, Ethanol FE of the Cu electrode modified by different molecules. FeTPPF[Cl], 5,10,15,20-tetrakis(pentafluorophenyl)-21H,23H-porphyrin iron(iii) chloride; FeTPPOMe[Cl], 5,10,15,20-tetrakis(4-methoxyphenyl)-21H,23H-porphine iron(iii) chloride; CoTPP, 5,10,15,20-tetraphenyl-21H,23H-porphine cobalt(ii). The error bars represent 1 s.d. on the basis of three independent samples. c,d, In situ raman spectra of the Cu (c) and FeTPP[Cl]/Cu (d) catalysts under different applied potentials. OCP, open-circuit potential. a.u., arbitrary units. e,f, Operando Fe K-edge XanES (e) and EXaFS (f) spectra of the FeTPP[Cl]/Cu catalyst under different applied potentials. Dry sample 200 400 600 1,800 2,000 2,200 200 400 600 1,800 2,000 2,200 0 2 4 6 x (Fig. 4a); the ratio of ethanol to ethylene increased from approxi- mately 0.5 for pure Cu to approximately 1 for the FeTPP[Cl]/Cu. The ratio was also larger than the values previously reported for Cu catalysts (Supplementary Table 1). These results were consistent with DFT calculations, in which high CO coverage favoured the ethanol pathway over the ethylene pathway. To explore whether the local high-concentration CO directly contributed to the enhanced ethanol selectivity, we modified the Cu electrode with the molecule Ni-tetraphenylporphyrin (TPP; NiTPP), whose CO2-to-CO conversion onset potential was more negative than that of the FeTPP[Cl] molecule (Supplementary Fig. 20). We found that only after the NiTPP started to generate CO did the ethanol FE show marked improvement on the NiTPP/Cu catalyst (Supplementary Fig. 20). This agrees with the view that improved ethanol selectivity arises from local CO enrichment. We further investigated the role of the FeTPP[Cl] molecule by varying its loading on the Cu substrate. We found that both the combined C2 FE and the ratio of ethanol to ethylene gradu- ally increased against the surface coverage (see quantification details in Supplementary Note 3 and Supplementary Fig. 21) of the FeTPP[Cl] on the Cu and reached the maximum values when the coverage was near 1. This trend suggested that the locally concen- trated CO provided by the FeTPP[Cl] enhanced the ethanol selec- tivity in CO2RR. A further increase of the FeTPP[Cl] coverage led to decreased ethanol selectivity, which we accounted for by noting that too few Cu sites were available for C–C coupling, in agree- ment with the observed increased CO FE. We highlighted that the additional layer of molecular catalyst on Cu allowed the ratio of *CO-producing sites to C–C coupling sites to approach unity. In contrast, the heteroatom doping strategy was limited to an analo- gous ratio of approximate 0.02 in the Au/Cu bimetallic catalyst27. We noted also that the *CO-enrichment strategy was expected to substantially leave the electronic properties of Cu unchanged, in contrast with the elemental alloying approach29. We carried out control studies to examine whether ethanol selectivity was influenced by the porphyrin framework itself. Using in situ Raman spectroscopy, we found that the TPP frame- work did not tune the binding properties of the Cu substrate to CO (Supplementary Fig. 22). We found no enhancement of ethanol selectivity for the TPP/Cu electrode (Fig. 4b). These observations NATurE CATALYSiS | VOL 3 | JanUarY 2020 | 75–82 | www.nature.com/natcatal 79 Fe reference Fe2O3 reference Dry sample –0.52 V –0.61 V –0.82 V 7,140 TPP FeTPP[Cl] FeTPPF[Cl] FeTPPOMe[Cl] CoTPP Intensity (a.u.) FT [k3χ(k)] Intensity (a.u.) FEethanol + ethylene (%) FEethanol (%)PDF Image | Cooperative CO2-to-ethanol conversion via enriched intermediates
PDF Search Title:
Cooperative CO2-to-ethanol conversion via enriched intermediatesOriginal File Name Searched:
s41929-019-0383-7.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |