
PDF Publication Title:
Text from PDF Page: 012
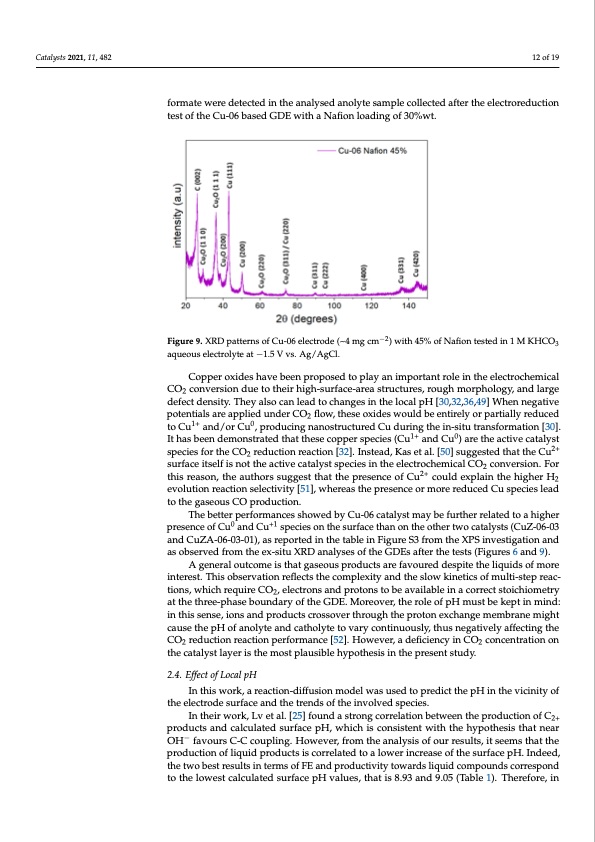
Catalysts 2021, 11, x FOR PEER REVIEW 12 of 19 Catalysts 2021, 11, 482 12 of 19 mmol h−1 gcat−1) by increasing the Nafion content from 30% to 45%, which could be also attributed to the presence of high-index facets of Cu, such as (400), (331), and (420). Indeed, in a recent work, Philip et al. [45]have demonstrated the suppression of H2 evolution due formate were detected in the analysed anolyte sample collected after the electroreduction to the increased amount of these high-index Cu facets. test of the Cu-06 based GDE with a Nafion loading of 30%wt. −2 FiFgiugruere9.9X. RXDRDpaptatettrenrsnosfoCf uC-u0-60e6lecletrcotrdoed(e~(4~m4 gmcgmcm) w)itwh i4t5h%45o%f NofafNioanfitoenstedstiend1inM1KMHKCHOC3 O aqauqeuoeuosueslelcetcrtorloyltyeteata−t1−.51.V5 vVs.vAs.gA/Ag/gAClg.Cl. Copper oxides have been proposed to play an important role in the electrochemical 2.3.5. Considerations about the Obtained Results CO2 conversion due to their high-surface-area structures, rough morphology, and large Some considerations about the apparent inconsistency of some results should be defect density. They also can lead to changes in the local pH [30,32,36,49] When negative done. Indeed, as can be observed, in some cases, the overall FE exceeds 100%, while, in potentials are applied under CO2 flow, these oxides would be entirely or partially reduced other cases, this value is less than 50%. It has also been observed in previous studies by to Cu1+ and/or Cu0, producing nanostructured Cu during the in-situ transformation [30]. different authors [46,47]. A possible cause could be the experimental uncertainty in meas- It has been demonstrated that these copper species (Cu1+ and Cu0) are the active catalyst uring the inlet and outlet real flow rates of the gaseous streams. A slight variation of these species for the CO2 reduction reaction [32]. Instead, Kas et al. [50] suggested that the Cu2+ values might significantly impact the processed data, with particular reference to the effi- surface itself is not the active catalyst species in the electrochemical CO2 conversion. For ciencies beyond 100%. When FE is far below 100%, a possible reason may be the oxidation this reason, the authors suggest that the presence of Cu2+ could explain the higher H2 of liquid products that travel to the anolyte through the membrane. As suggested by evolution reaction selectivity [51], whereas the presence or more reduced Cu species lead Gabardo et al. in their work, a control experiment based on the verification of the oxida- to the gaseous CO production. tion levels of possible products in the anolyte can be performed [48]. Otherwise, analysing −2 3 The better performances showed by Cu-06 catalyst may be further related to a higher the distribution of the liquid products in the two liquid chambers of the flow cell would presence of Cu0 and Cu+1 species on the surface than on the other two catalysts (CuZ-06-03 allow having important qualitative information. Relative to the latter case, traces of for- and CuZA-06-03-01), as reported in the table in Figure S3 from the XPS investigation and mate were detected in the analysed anolyte sample collected after the electroreduction test as observed from the ex-situ XRD analyses of the GDEs after the tests (Figures 6 and 9). of the Cu-06 based GDE with a Nafion loading of 30%wt. A general outcome is that gaseous products are favoured despite the liquids of more Copper oxides have been proposed to play an important role in the electrochemical interest. This observation reflects the complexity and the slow kinetics of multi-step reac- CO2 conversion due to their high-surface-area structures, rough morphology, and large tions, which require CO2, electrons and protons to be available in a correct stoichiometry defect density. They also can lead to changes in the local pH [30,32,36,49] When negative at the three-phase boundary of the GDE. Moreover, the role of pH must be kept in mind: potentials are applied under CO2 flow, these oxides would be entirely or partially reduced in this sense, ions and products crossover through the proton exchange membrane might to Cu1+ and/or Cu0, producing nanostructured Cu during the in-situ transformation [30]. cause the pH of anolyte and catholyte to vary continuously, thus negatively affecting the It has been demonstrated that these copper species (Cu1+ and Cu0) are the active catalyst CO2 reduction reaction performance [52]. However, a deficiency in CO2 concentration on species for the CO2 reduction reaction [32]. Instead, Kas et al. [50] suggested that the Cu2+ the catalyst layer is the most plausible hypothesis in the present study. surface itself is not the active catalyst species in the electrochemical CO2 conversion. For th2i.s4r.eEafsfeocnt,otfhLeoacaulthpHors suggest that the presence of Cu2+ could explain the higher H2 evo- lution reaction selectivity [51], whereas the presence or more reduced Cu species lead to In this work, a reaction-diffusion model was used to predict the pH in the vicinity of the gaseous CO production. the electrode surface and the trends of the involved species. The better performances showed by Cu-06 catalyst may be further related to a higher In their work, Lv et al. [25] found a strong correlation between the production of C2+ presence of Cu0 and Cu+1 species on the surface than on the other two catalysts (CuZ-06- products and calculated surface pH, which is consistent with the hypothesis that near − 03OaHnd fCauvZouAr-s06C--0C3-c0o1u),palsinrge.pHorotewdevinert,hferotmablteheinanFaiglyusries So3f ofruormretshueltXs,PiSt sineevmesstitghaattiotnhe production of liquid products is correlated to a lower increase of the surface pH. Indeed, the two best results in terms of FE and productivity towards liquid compounds correspond to the lowest calculated surface pH values, that is 8.93 and 9.05 (Table 1). Therefore, inPDF Image | Gas Diffusion Electrode Systems for the Electro CO2 Conversion
PDF Search Title:
Gas Diffusion Electrode Systems for the Electro CO2 ConversionOriginal File Name Searched:
catalysts-11-00482.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |