
PDF Publication Title:
Text from PDF Page: 004
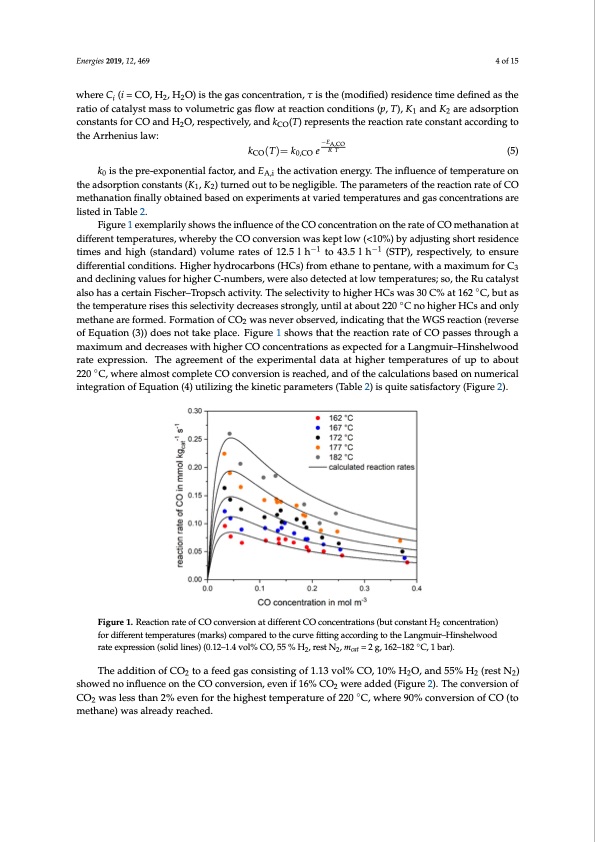
Energies 2019, 12, 469 4 of 15 where Ci (i = CO, H2, H2O) is the gas concentration, τ is the (modified) residence time defined as the Energies 2019, 12, x FOR PEER REVIEW 4 of 15 ratio of catalyst mass to volumetric gas flow at reaction conditions (p, T), K1 and K2 are adsorption constants for CO and H2O, respectively, and kCO(T) represents the reaction rate constant according to the Arrhenius law: kCOT= k0,CO e R T −EA,CO EA,CO (5) kCO(T)= k0,CO e R T (5) k0 is the pre-exponential factor, and EA,i the activation energy. The influence of temperature on the adsorption constants (K1, K2) turned out to be negligible. The parameters of the reaction rate of k0 is the pre-exponential factor, and EA,i the activation energy. The influence of temperature on CO methanation finally obtained based on experiments at varied temperatures and gas the adsorption constants (K1, K2) turned out to be negligible. The parameters of the reaction rate of CO concentrations are listed in Table 2. methanation finally obtained based on experiments at varied temperatures and gas concentrations are Figure 1 exemplarily shows the influence of the CO concentration on the rate of CO methanation listed in Table 2. at different temperatures, whereby the CO conversion was kept low (<10%) by adjusting short Figure 1 exemplarily shows the influence of the CO concentration on the rate of CO methanation at residence times and high (standard) volume rates of 12.5 l h−1 to 43.5 l h−1 (STP), respectively, to ensure different temperatures, whereby the CO conversion was kept low (<10%) by adjusting short residence differential conditions. Higher hydrocarbons (HCs) fro−m1 ethane to p−en1 tane, with a maximum for C3 times and high (standard) volume rates of 12.5 l h to 43.5 l h (STP), respectively, to ensure and declining values for higher C-numbers, were also detected at low temperatures; so, the Ru differential conditions. Higher hydrocarbons (HCs) from ethane to pentane, with a maximum for C3 catalyst also has a certain Fischer–Tropsch activity. The selectivity to higher HCs was 30 C% at 162 and declining values for higher C-numbers, were also detected at low temperatures; so, the Ru catalyst °C, but as the temperature rises this selectivity decreases strongly, until at about 220 °C no◦ higher also has a certain Fischer–Tropsch activity. The selectivity to higher HCs was 30 C% at 162 C, but as HCs and only methane are formed. Formation of CO2 was never observed,◦indicating that the WGS the temperature rises this selectivity decreases strongly, until at about 220 C no higher HCs and only reaction (reverse of Equation (3)) does not take place. Figure 1 shows that the reaction rate of CO methane are formed. Formation of CO2 was never observed, indicating that the WGS reaction (reverse passes through a maximum and decreases with higher CO concentrations as expected for a of Equation (3)) does not take place. Figure 1 shows that the reaction rate of CO passes through a Langmuir–Hinshelwood rate expression. The agreement of the experimental data at higher maximum and decreases with higher CO concentrations as expected for a Langmuir–Hinshelwood temperatures of up to about 220 °C, where almost complete CO conversion is reached, and of the rate expression. The agreement of the experimental data at higher temperatures of up to about calcul◦ations based on numerical integration of Equation (4) utilizing the kinetic parameters (Table 2) 220 C, where almost complete CO conversion is reached, and of the calculations based on numerical is quite satisfactory (Figure 2). integration of Equation (4) utilizing the kinetic parameters (Table 2) is quite satisfactory (Figure 2). Figure1.ReactionrateofCOconversionatdifferentCOconcentrations(butconstantH concentration) Figure 1. Reaction rate of CΟ conversion at different CO concentrations (but 2constant H2 for different temperatures (marks) compared to the curve fitting according to the Langmuir–Hinshelwood concentration) for different temperatures (marks) compared to the curve fitting according to the rate expression (solid lines) (0.12–1.4 vol% CO, 55 % H , rest N , m = 2 g, 162–182 ◦C, 1 bar). Langmuir–Hinshelwood rate expression (solid lines) (02.12‒1.4 2vol%catCO, 55 % H2, rest N2, mcat = 2 g,162 −182 °C, 1 bar). The addition of CO2 to a feed gas consisting of 1.13 vol% CO, 10% H2O, and 55% H2 (rest N2) showed no influence on the CO conversion, even if 16% CO2 were added (Figure 2). The conversion of The addition of CO2 to a feed gas consisting of 1.13 vol% CO, 10% H2O, and 55% H2 (rest N2) CO2 was less than 2% even for the highest temperature of 220 ◦C, where 90% conversion of CO (to showed no influence on the CO conversion, even if 16% CO2 were added (Figure 2). The conversion methane) was already reached. of CO2 was less than 2% even for the highest temperature of 220°C, where 90% conversion of CO (to methane) was already reached.PDF Image | Selective Methanation of CO over a Ru-y-AI2O3 Catalyst in CO2 H2
PDF Search Title:
Selective Methanation of CO over a Ru-y-AI2O3 Catalyst in CO2 H2Original File Name Searched:
energies-12-00469.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |