
PDF Publication Title:
Text from PDF Page: 007
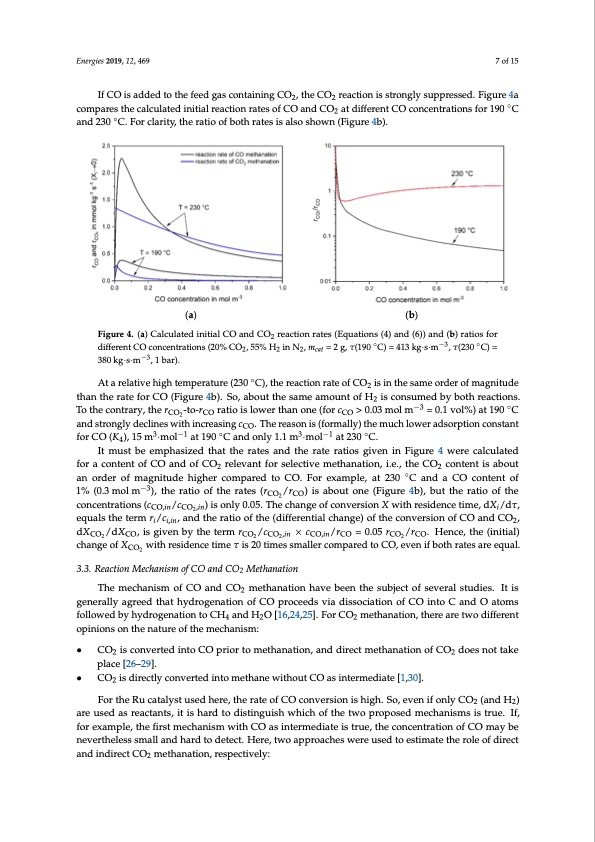
Energies 2019, 12, 469 7 of 15 If CO is added to the feed gas containing CO2, the CO2 reaction is strongly suppressed. Figure 4a compares the calculated initial reaction rates of CO and CO2 at different CO concentrations for 190 ◦C ◦ −3 −3 3k80g·ks·gm·s·m, 1 ba, r1).bar). Atarelativehightemperature(230◦C),thereactionrateofCO isinthesameorderofmagnitude At a relative high temperature (230 °C), the reaction rate of CO2 2 is in the same order of magnitude than the rate for CO (Figure 4b). So, about the same amount of H is consumed by both reactions. than the rate for CO (Figure 4b). So, about the same amount of H2 i2s consumed by both reactions. To aEnnder2gi3es020C19., F12o,rxcFlOaRritPyE,EtRheRErVatIEioWof both rates is also shown (Figure 4b). 7 of 15 (a) (b) FFigiguurere4.4.(a(a) )CCaalclcuulalateteddininitiitaial lCCOOaannddCCOO2rreeaacctitoionnrraatetess(E(Eqquuaattioionnss((44))aanndd(6(6)))aanndd(b(b) )raratitoiossfofor r 2 ddififefreernenttCCOOcocnocnecnetnrtartaiotinosn(s2(02%0%COCO,25,5%5%HH2ininNN,2,mcat=2g,𝜏τ(190°CC))==441133kkgg·s··sm·m, 𝜏,(2τ3(203°0C)C=)3=80 2 2 2 cat ◦ −3−3◦ To the contrary, the r -to-r ratio is lower than one (for c > 0.03 mol−m3 −3 = 0.1 vol%) at 190 ◦C the contrary, the rCOC2-Oto-rCO CrOatio is lower than one (for cCOC>O0.03 mol m = 0.1 vol%) at 190°C and 2 and strongly declines with increasing c . The reason is (formally) the much lower adsorption constant strongly declines with increasing cCOC.OThe reason is (formally) the much lower adsorption constant 3 −1 ◦ 3 −1 ◦ forCO(K ),15m 3·mol−1 at190 Candonly1.1m3 ·mo−l1 at230 C. forCO(K44),15m·mol at190°Candonly1.1m·mol at230°C. It must be emphasized that the rates and the rate ratios given in Figure 4 were calculated It must be emphasized that the rates and the rate ratios given in Figure 4 were calculated for a for a content of CO and of CO relevant for selective methanation, i.e., the CO content is about content of CO and of CO2 relev2ant for selective methanation, i.e., the CO2 conten2t is about an order an order of magnitude higher compared to CO. For example, at 230 ◦C and a CO content o−3f ofmagnitudehighercomparedtoCO.Forexample,at230°CandaCOcontentof1%(0.3molm ), 1% (0.3 mol m−3), the ratio of the rates (r /r ) is about one (Figure 4b), but the ratio of the the ratio of the rates (rCO2/rCO) is about one (CFOigurCeO4b), but the ratio of the concentrations (cCO,in/cCO2,in) 2 concentrations (c /c ) is only 0.05. The change of conversion X with residence time, dX /dτ, is only 0.05. TheCcOh,ianngCeOof,inconversion X with residence time, dXi /dτ, equals the term ri /ci,in, anid the 2 equals the term r /c , and the ratio of the (differential change) of the conversion of CO and CO , ratio of the (diffeireni,itnial change) of the conversion of CO and CO2, dXCO2/dXCO, is given by the ter2m dX /dX ,isgivenbythetermr /c ×c /r =0.05r /r . Hence,the(initial) rCOC2O/cCO2,in×COcCO,in/rCO=0.05rCO2/rCO.HeCnOce,tChOe(,initialC)Och,inangCeOofXCO2wiCtOhresCidOencetimeτis20times 2222 change of X with residence time τ is 20 times smaller compared to CO, even if both rates are equal. smaller comCOpared to CO, even if both rates are equal. 2 3.3. Reaction Mechanism of CO and CO2 Methanation 3.3. Reaction Mechanism of CO and CO2 Methanation The mechanism of CO and CO2 methanation have been the subject of several studies. It is The mechanism of CO and CO2 methanation have been the subject of several studies. It is generally agreed that hydrogenation of CO proceeds via dissociation of CO into C and O atoms generally agreed that hydrogenation of CO proceeds via dissociation of CO into C and O atoms followedbyhydrogenationtoCH andHO[16,24,25].ForCO methanation,therearetwodifferent 422 • CO2 is converted into CO prior to methanation, and direct methanation of CO2 does not take • CO2 is directly converted into methane without CO as intermediate [1,30]. For the Ru catalyst used here, the rate of CO conversion is high. So, even if only CO2 (and H2) followed by hydrogenation to CH4 and H2O [16,24,25]. For CO2 methanation, there are two different opinions on the nature of the mechanism: opinions on the nature of the mechanism: CO2 is converted into CO prior to methanation, and direct methanation of CO2 does not take place [26–29]. CO2 is directly converted into methane without CO as intermediate [1,30]. place [26–29]. are uFsoerdthaesRreuactatnatlsy,sittuisehdarhderteo,dthisetirnagteuiosfhCwOhiccohnovferthsieontwisohpirgohp.oSsoe,demveenchifaonnislmysCiOs tr(uaen.dIfH, fo)r 22 aerxeaumsepdle,asthreafcirtastntms,eicthiasnhisamrdwtoitdhisCtiOngausisihntwerhmicehdioaftethies twruoe,ptrhoepocsoendcemntercahtiaonisomfsCiOs trmuae.y Ibf,e fonreveexratmhepleles,stshme afilrlsatnmdehcahradntiosmdewteictht. HCOerea,stwinotearmpperdoiactheeis wtrueree, tuhsedcotnocesntitmraatitoenthoef rCoOle mofadyirbeect naenvderitnhdeilreescstsCmOa2llmanetdhahnaardtioton,dretsepcet.ctHiveerley,:two approaches were used to estimate the role of direct a1n.d inTdhireercat tCeOof mRWethGaSnacatinonb,eresptiemctaivtedlya: nd compared with the measured CO2 rate based on the 2 (easily) measurable rate of the reverse WGS reaction and some thermodynamic considerations. 2. The rate of CO2 conversion must equal the rate of RWGS, if (hypothetically) only indirect CO2 methanation via CO takes place. The RWGS is then the rate determining step followed by fast CO methanation. A calculation based on the CO and CO2 rate equations (as determined in this work) lead to the concentration of the intermediate CO, which should correspond to thePDF Image | Selective Methanation of CO over a Ru-y-AI2O3 Catalyst in CO2 H2
PDF Search Title:
Selective Methanation of CO over a Ru-y-AI2O3 Catalyst in CO2 H2Original File Name Searched:
energies-12-00469.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |