
PDF Publication Title:
Text from PDF Page: 224
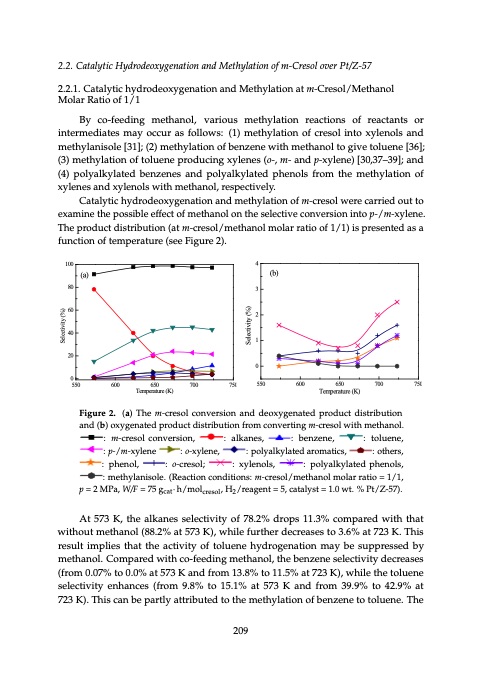
% Pt/Z-57). cat cresol 2 11 11 00000 0 550550550 5550560060065050 6060060506506500 6560570070076050 7 2.2. Catalytic Hydrodeoxygenation and Methylation of m-Cresol over Pt/Z-57 2.2.1. Catalytic hydrodeoxygenation and Methylation at m-Cresol/Methanol Molar Ratio of 1/1 By co-feeding methanol, various methylation reactions of reactants or intermediates may occur as follows: (1) methylation of cresol into xylenols and methylanisole [31]; (2) methylation of benzene with methanol to give toluene [36]; (3) methylation of toluene producing xylenes (o-, m- and p-xylene) [30,37–39]; and (4) polyalkylated benzenes and polyalkylated phenols from the methylation of xylenes and xylenols with methanol, respectively. Catalytic hydrodeoxygenation and methylation of m-cresol were carried out to CaCtaCltyasltytasCsltCy2ass0at2ta1ts0al6y21lC,sy06t6sa1s,tt6s62a,20ly601s16ts6, ,626016, 6 examine the possible effect of methanol on the selective conversion into p-/m-xylene. 44 444 The product distribution (at m-cresol/methanol molar ratio of 1/1) is presented as a ExEcxeEcpxetpcetaEplExkatxcalkencaapelknptsEae,txnsacac,laekerelsoakap,rmnatoaneamarasetol,lsiakmc,tasianrcna,otsreioam,scsnm,sad,tnaitradrcpionschm,dspe,hanpetnaoihncdcleiosdcn,lpsioc,phalshenio,cnedtsnoho,oletpihlrochietscehrp,sner,oporlortdihopctuhedsrscroe,utrsdcoputstptrschourteodscruduhcsucpuhtcarsrctsoshadsiusnaucdishtctnashdninaessdanu,saecin,hniaendnp,adahsnanptnehahi,epatnh,lhdneatanahlnenpaeanep,hl,e,htanh,tnheaad,la Catalysts 2016, 6 ocththseerrsuppcrrohodduauscctstisnsdsuaucncheh,aanssapinihndtdhaannle,n,enn,aapaphnhdtdhtehadratliehelvdreneaienvietreia,iv,vtdeiavdeasntre,nidivsradvi,elvastasthadtdo,lihtseveaieoirvelrixiserveio,sxa,ateiiliasixvsvtnloiseisosnstem,xiesanxaimlslsistlsaomtilanleimanaxlslomimsuamtoamniuilatnlosnll.autssmamn.mtoaso.ullunantmsmt.so.unts. 5 (b) 100 55 5 5 4 (a) 33 33 2 40 22 22 4 (b) 3 2 1 0 6076050605065065005506560570070076050 6070070070507507500 55555 5 (b)(b)(b) (b()b) (b) 44444 4 33333 3 22222 2 11111 1 function of temperature (see Figure 2). sic,so,tohethrerprpordoudcutsctsuscuhchasasindinadnaen,en,anpahpthathleanlen,ea,nadndthtehireir (b) 80 (b) (b)(b)(b) 80 80 80 8080 80 4444360 606060606060 1 20 00 000 0 550 404040 4040 40 20 20 20 20 20 20 000 00 0 650550550550 575505600060065050 100100100 101000 100 5 (a)(a)(a) (a()a) (a) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity (%) 750 700 FigFuigreure2.2.(a()a)TThheemF-i-cFgcruiregFersuiseogrolu1elF.rFc1iceEgo.iogufn1Enfurv.evfereFcEferti1ifrscgfo1.siteuof.iEcornEtrfenefoearf1a1feacecn.atctnrdiEcEetodtafonfifcfodfedetrnteicecereotatemtonxceaoxptmcyftfiyteogiprmgoneaenenatprtunacetrtameruieiamotordptenoenupednreotpeaern(prtamaourt()nudrpopaerueted)(ehrcaoueatato)hntcuncetd(orhraece(idnesa)ovioto)cnstrenhoivtrbrhense(iueivrabocste)ioucniorontstnhnhainivoeneveandraecncsrnoadisodnieodnvdvonexadorynasexgnidioyoedxgndyedaeagnatoeneneaoxdntdxyeaygdpdtgerepodneordnaxoputaydertcgodeu 600 657057050 Temperature (K) TemTpeemrTapteumrraeptue(rKrTeae)tT(umKerepm)e(prKaetr)uaTrtTueer(meKp(p)Ker)ature (K) Temperature (K) TemTpemeTrpaeetmurarpeteuT(rKaetm)u(Krpee)r(aKtu)Trem(Kpe)rature ( Temperature (K) 600550 650600 550 550550 600 600600 650 650650 700 700700 750 750750 00757050 750750750 550 Temperature(K) TemperaturTee(mKp)erature(K) 700650 600 650 700 750 TempeTraetmTurepeme(rpKaet)uraretu(rKe)(K) 750700 750 eocononvne(v(areas)ri)sotihtnoheneacacnonodndnvvederersosioixonynaganeandndad(tdbeded)eoopxpxryryoygodgeduenuncnactatatettededdpidsprptirorsdoridtbidorusdiudtcbirtctsuiuitobttcrniuditobtdinidsuioatsitrnantsirondbtiarndbdudniuitibad(sistbountir(on)dtibinboa)(uxbnanotyndi)ixofgdoryn(neobxg(nabmay)eang)ntodeacoxdntoxy(eabnygdpt)gverpodneoeordnarxoputatyderictgngoduetedgcdpnutiprmacsdortie-oedrscdidutbrirucesuiptcbtstrtiuodoiobtdidldnisuiouwtstrncfctirirotbotifnbhdudrmouitifsismtrotmioroneim-mnbtcfhur-fmotectarisormno-eocmnoslrmolefH.mlsr-ocDH-mlrcOeDrHse.mOosDol-.OclHrH.eDsDOo:Ol.mH.: -DmDc:rO-Omec.sr-eoc: truatrueroenon(a()at)hethceocno(vnbev)resoiroxsniyognaenadantdeedoepxoryoxgdyeugnceatntdeaditsetdprirbpourdotuidocutncftrom converting m-cresol with methanol. : m-cresol ondudficfsriurtsotocrtmdrimtbibdsumctuitrosit-iotinbcronrviunbetfsiurfrotroinlonomgHnfrmDofmrm-Ococ-comc.rmnereesvs-mosoceol-rlcewsHrsiHoieoDt:snhDlomO,mHOml-.DH-c.ecrtODheresa.Oosnolo.:l:.a:cmlmokc-na-c:ocnrcvnmre:oeves-scnmrseoc:,ovsorl-rmniselceoivrs-ocsnecoeocnisrl,one,osnsivlo,:venlrebc,sroeisonionvz:n,ea,rnr:aslelkaik:,oalkanaan,leknse:ae,s:nas,,laekls:ka,nta:onela:seul,lsbke:,eanabnbe:eze,ebnesnez,nzne:enze:,benben,ene,nz:,:ezpneb-ne:/,emnt,:zo-exlt:uo:nyeletluton,e,oelen:unl,eu:etoen,tolneul,eue:,:nenet:o:,el,pu:-ep/n:m-e/p-m,x-/-y:mxl:pey-pxn-l/e-yemn/lme-:xn-pxye-yl/elmn nzze:en:nteoeb,l,euneznen,e:,:totolulue:ennpe:e,-t,/omlu-xeynlee:,:n:pep-p-/-/m/m--xx:y:ylplele-en/nmeo-x-oxy-ylxeoleyn-:xnle:eyeono,l-eo-xn,-yxyely,le:lnonoen-e-:x,ey,p:loepl:nyoepal,yolka:l:yl:pkplaayopoltkloleayldtyal:e:alaldakpktplerkyoyoadylrmayaloatatamerteltodekdiadmcytasialarca,arotsrtoieoem,cmdmsa,ataitrciosc:m,so,:atthoti:etchorsse,t,hr:se:,orosot,hthere:sr,sops:,t,thpe:ehnrpeoshn,le,o:n:lp,ophlhe, ne:n:o:olp,ol:h,-eoc:nr-oeocosrl-,e,oc:slr b:ebnezneznen,e, : t:otlouleunen,e, : p:-p/m-/-mx-yxlyenlene : : o-xylene, : polyalkylated aromatics, : others, : phenol, : o-cresol; mcaocsrtams,oht,imeacrtsiasc,tsic, s:,:ootht:hepeor:rhsts,oh:e,tenohroteshl,r,es,rs:,:pphhe:enpn:opoho-lph:ce,lhe,rnpenohsnloeo,nl,;o, :l,:oo--c:crreo:es-soc:ol-r-o;lec:;-srceoe:xrslsey;oxo:sloey;l;xnl;eyon:l:eso,xnlsxyo,ylesl::,nenoxolysyll,sle:e,n:poolp:slsyo,palyolk:al:ylkplaaypoltkloleay:ldt:yaleapladkptploekyohdlpyleyayhlnatapeotlhndeklseody.nllplsaoap.h(tlRthese(.dnedeRdnoa(eolcpRpsatlh.iscehoe.taen(incoRn(otnRielolcosaeol.n.scan,otcd(itcnRoRiotdoneininoatdnicoistoctini:ionosndn:npdistip:ict=oionpn=nsd:s2=i:tip2ioMpn2M=Ps=: : xylenols, : polyalkylated phenols, : methylanisole. (Reaction edyda(Rlpkpheyhaelecnantoieoldslns.. p(c(RhoReneneadaocictltisitoi,onns:ccoponnd=di:ti:imtoio2nmenstes:hM:thypPylpalan=,=nisiso2oWl2leW/.M.FM(/WRPF=(PRae/a=7,Fae,5Wca7=Wtcg5i/to7Fci/agoF5nt·c=nahg=ctW·/7comha75/tn/·F5omhdgl/g=coimartleti·7csohor·5elnh/,scmogrg/sHlem,c:csoaoa2Hmtotl/·,clh2hrH-re//csecmo2rsrl/,eocos,sHlrloceHc=2rlsle//sosc=5o/olrcml,,e5r=cHsHe,eaos5ct2t2olha/,/acl=ltacyra=anes5ltysto5a,os=l,ctlylacm=s1=tata.ot015l=ayl.,law0ys1crtst.aw0.t=rta%at=w.1lty%i1.tPo0s.s.t0t%P=w/=Zwtt/1P-.1Zt5/.t%.-/701%Z5),w7w.P-5)Pt./7.tZ/)%Z.-5-5P7P7)t/./)Z. -57). yklaytleadtedphpehneonlso.ls.(R(eRaecaticotnioncocnodnidtiiotinosn: s:p p= = 2 2MMPaP, a, cat cresol 2 conditions: m-cresol/methanol molar ratio = 1/1, p = 2 MPa, W/F = 75 gcat·h/molcresol, m0a=5aotwa,tl5alactyl,ra.ysct%staratal=tya=tPils1oty1t/.sZ0.=0t-w=15w.7t101.t)/..%1.w0%, twpP.Pt%/=.tZ/%pZP-2-5=t5P/7Z72Mt)/).-ZM.5P-7a5P),7.aW),.W/F/F==775 gcat·h ̈/hm/omlcroeslol, , H /reagent = 5, catalyst = 1.0 wt. % Pt/Z-57). H2/reagent = 5, catalyst = 1.0 wt. % Pt/Z-57). GeGneGnreaenlrlaeGylrG,leaynelt,lhneyetre,haGreclatlhealylcnenty,ae,ltctryahattlheltiylaceytlci,y,cHacttatiDhahHctlaeOyDlHytcOitDcaoaictfOaoHloHfyDoxtDoifiOycxOgoyeHoxgnofyeD-fgncoOeo-oxcnxyo-otygacnfgeiotnaneonoin-xtxnca-ygiocingngongceientnonantcgiam-nocinipcmoniongpmutgoancpiudnoconsiomudnmnsgugpdnpoucscdounoeunudmrndnedspdrsoieufdurfuninenfndrfdeiesfrenrferetudnrndcietfdoinfcenetforedcneridonetdintioftdtfniceoisoctr comntpaoinuindgscuonmdperoudnidffseruendtecrodnidfifteiorenndstifucfseodirneidfgnfietdiroiecfnafstetarleucydnassitftfnasflgceyarisesettanslktyniscscotaswtktanilsyoystwskotsnsnopiwtrsonckpenterodwcpentnrehodrtcoeutehgpdrhrooutctchgwerheoeodutgrwtethoarctotwruiueoganhchtripetotawawnctohotipworarnaetyhaspcwctatiai[to3yhn4sw,a3p[y35as4]th.,3w[wT35a4h]y.,e3sT5o[]hn3.ee4T,o3ph yeogne-tnca-oincnoitnangitnaicnoigmngcpocmuonpmdopsuonudnsdesurnudneidfrfedrriedfnfietfrfcedorniefntfndectirotecinodtnisdtfcifioauettinrsaoesilnysgutsstuscinsaigitnsalgyksntoswisn ktonopwrnocetoedprtohcroeuedghthtrwoougrheatcwtionrepaacthiownaypsat[h3w4a,3y5s].[3T4h,3e5]o.neT At 573 K, the alkanes selectivity of 78.2% drops 11.3% compared with that without methanol (88.2% At 573 K, the alkanes selectivity of 78.2% drops 11.3% compared with that 7odeut8auhg.cgrt2hoth%iruotgnwtudwhgorpohtapwrtrestheowa1wac1octrai.tey3oiraos%necnat[icpc3optoa4inatom,ht3nhpwp5wa]aptr.ayhaeystwdThshaw[ye[3ai3s4ty4ho,s3,[nt35he5[4]a3].,htp.34ywTa5,hT3dt]hiyh5.rhtehdho]eyT.rogisoduehoTngrntehnoehomhyegneloydeypoendprntsaloheyraiotnosghatshlehgeinpyoysneoasirdpsioniotlsdrsrharol(oiyto8lrdhgysier8iseiscr.dsniei2tsioc%drtolerleycdrdostedixirdosiyerexogceoyrtrcexgtndyedaeignrtoeieaoxocntxynitaoygdt(ngieDeoneo(onDnaxta(yOyDiDtogi)OoneDeon)(OfaDao(ptD)fiDihopDeOnfhnOp)e(oDhn)olesofDnlftpsOoOophtl)pheosrneotponofrdolpopsulhdrsctoeuoetndncopaouoeprlcloasreromdtoadouoamrucptopeciarmrcetoaihadrdcaotyruihomdcymrehadotayarciotrdcaiocrhmboahyrocabydantodrisocbcnr.ocosThac.nyhraTsdbd at 573 K), while further decreases to 3.6% at 723 K. This result implies that the activity of toluene without methanol (88.2% at 573 K), while further decreases to 3.6% at 723 K. This )3to.of6fp%pprhoheadentunoc7oles2ls3atotroKpmpr. roaTodtdhiucuicscheeyraedarsrouomlmctaairtmitbciocphnlhyisey.dsdTrrothohocecanoatroerbntbohoiehsoneneinrestshs.ao.ietcTshonTthicenethveoheiciuetosoiyoptsocuthtlnhohoepteheuedflrerpiticdrsolsiocenlroutdtugihupneerplngsielanecdestgoduaururtsiurpapnaitrlntgieauogdtrsniaoas/rtrtnaiuanot/uprgnari/dtpasrsitaiaoaidptonuein/drhr/aeayrtdthpadieypoiordhdinandytr/diradodaeatrniphpeao/hiyithndiydoy/drhdndarye/rtahdhoihtoyriygyonodedn/grnrho/eahygntyidieaodrntnonirao/oghthn(gieyHyone(dnYaHrtao(DiYtHogi)oDneYnt)(oDaH(tH)opiYortYpnonoDrdD)o(puH)drtcouYteodcpDcuepryc)ocrecodytlodoucuypclpoecacrperlo DOD)Oo)fopfhpehneonlosltsotporpordoudcuecaeroarmoamtiactihcyhdyrdorcoacrbarobnosn.sT.hTehoethotehrer hydrogenation may be suppressed by methanol. Compared with co-feeding methanol, the benzene result implies that the activity of toluene hydrogenation may be suppressed by dorramgateiteointonhan/tahi/nohyonydld.r(roHoCggYeoenmDnaap)titaotironendp(r(HoHwYdYiutDhDc)e)ctocotoy-fpceplreorodopdidaunTurcgacheTfefmhciTcnesyeyehsctsc.cheleolaTocspnTpoheadochanerloraed,pasfnafeftspdThtficehnaiehocnptseohenis.abn.ndtsesvihdnenopcvczilpaonovoetanvnhenltvdsohdeeilntvspiphnveaetvosththoleivtnlhvieitinesnevrstitmeonhtrlthemvedereiimsnadinteiteahthdretemiepramriteponederdtdioepuairdrtcamoeutdecepcutdyprcicocratyodltoeceduhylucopcetchrlxtoceaydcdhxnycueaolcxncloltaohonlcheroyoxelcscxraulonaosbrnouhsolbesetliuxsxottbuaoarintstnresutoiudsttleubudcobosteytrsricdctiysultuocutebhyltodesechdtxliocetayucuhxnycteaoelcxnlodla.ohn edheyhdyrdatriaotino/nh/yhdyrdorgoegneantiaotinon(H(YHDY)Dt)otoprpordoudcuececycyloclpoaprafrfaifnfisn.s. selectivity decreases (from 0.07% to 0.0% at 573 K and from 13.8% to 11.5% at 723 K), while the 0oeaho.dt0edpu%xurcpoactrndtaocoutcdycly5uctoc7cloclrt3oyhschceKuyelxbocxaslhanotneniohdtxoulealtxfoneroarodonrlmsocsuolyubr1cbos3lsrto.iut8tihsbut%ueustbxeteidsatdtonuictoceu1ylydt.1csecl.Itd5oclunsoy%hdtchucetysyheldxt,oacxiuyasthltadn,ohen7ysohetxot2sh,lerua.tl3extu.dnhrIadyoIenKiensny,lro).sit,et,ushtlhIuhut.ninwiheusdidsdIsrenhynyterhudi,teltineietshtscdthiiesteseauhctrbuntealncdebtdieialsteabetmulceaentcomadtuabaeoemnbltuetelonecoautmafanombtomfloeoueemufntanhtemtytohelofcytufhylmncycmytlelocteohyhltfohycehylxlmocelaychxenyctetaohlcxnloylaohlinlcnheoyixelnctcxahlinaoetnoholepetehlrxipoenairdnoptuohdrtclhoeutedscipn,nutsprcio,trmthodsiepm,duluciypmtcilrsrtnyo,psgid,linyumuigtimchnptatgspslhty,laytitihnthimnaget methanol. Compared with co-feeding methanol, the benzene selectivity decreases toluene selectivity enhances (from 9.8% to 15.1% at 573 K and from 39.9% to 42.9% at 723 K). This %ynemlclotchyeltytyochiclnoycl1oyhl5tchceh.y1elxeocx%alhapnoenroahxoltealdx5inunia7o(ncn3fltorshtiKoh,lenemimanptphnrproted0hold.yedpf0uirurn7copocgtm%rdstos,ut,dh3citumia9tomsct.,p9t0pstl%ih,y.lm0yreieni%tpmnoarDglegcpyD4atartliicehy2ntotOhtai.gni9na5cto%tgtrt7rnitehoroht3aetarhunaehocatetKtatceurDit7totDohmier2aDuonteDh3tnanmaOaereDiOcdoKnramtoDuDli)ynofouta.ODerlntnioTyeonOmcrhlmomocyaicusuiaocnrt1iuecsnl3ycrldmsyu.uo8radaorcs%iuicndruclgiuytunrrosrtgoiohsndc1etgduhc1mureuti.rhrn5-misecng%r-dgmectuhsrt-arehoecitselnrmoeg7Hgmls-2oDtctH-h3lrcODerHKsemOmopsD)olr-,OpoclHrwrcHoeDpescDrshOoesOilsclupseHenprsuDoDdsrtnocheOudecrenesetpdrshtrseotuohrunlctetuendehstdsesetsrenstrdtutuehtnscdhetdoectendeortrdsecntishoetdtiedeniotdnitcoeisoctsnsion(tosepndnd(dis=ptict(o=i2op can be partly attributed to the methylation of benzene to toluene. The methylation of toluene could be osiocoelnsHsoDuf nObdeepnrzoethcneststesosuetneloedlecucrteointvnhedei.tiTtyteihosenteesnmd(hpecatoh=nydW2cliaet/MtiFsioWPn=n(saf/,oFr7(opf5W=mt=go/F7cl2au5t9=·eMhg.WnW8/7cemPa%/5/tFa·coh,gol=/cutmaroelt·7sdoho5l1/,bcmrg5eHecso.aoa21tl/·,c%hrHre/semo2sla/,octHlrlce=r2r5se/so7co5olrl3),e.=HsHTKo52hl/)ce=.arTen5shf)doe.lrlrTe=f=,hfroaeo5rlr)mke.,afTonahrh3elekes9,ar.iena9nfle%okostrahreinen,tepoasthrlkikoe4nad2pnuth.rec9oestsd%ipunmrcotatahdsyteumbcptaersryogmdbeuanecyegtrsbeaetnmedgareyafntreboe mocl-rceHrseoDsloOHl DpHrODocOpersposrcuoencsedsseusrnutdhnedretterhsetthetedestcetoesdntedcdiotciWnodn/iFstdii(o=tpnios7=Wn5(s2p/gF(M=pca=t=·P2h7aM2/,5mMPgoaclPac,tra·eh,so/lm, Hol2c/rcesroel,soHl2=/cr5e)s.oTlh=er5e)f.oTreh,earlekfaonre,sailnkathnesprinodtuhcetsprmodauycbtsemgeanyebraetegden confirmed by the change in the xylenes selectivity, i.e., the p-/m-xylene selectivity (a maximum of 24.0% essfeoliernec,tihaveliktpyar,noie.desu.i,cnths7teh2mep3a-p/yKmrob)-dex.uyTgclehtsniemsrsacaetyaelendbcetfbirhvgoeyiemtdnpyrehta(orhaygraedmteelrnhydoayxgtfadieromrtonontmaurghthomieybyoftnhdnoutaeorftotoli2euofg4denet.on0oelta%ufo.teitontnhleu.eoefnmeto.elutehenyel.ation of benzene to toluene. The hfToehre,rfoeafrloekr,aena,lekaslakinaenstehisenipnthrotehdpeurcpotrdsoumdcutascytmsbameyagybeenhbeygerdeagrntoegdnreahfntryeaodtmeirodforngtofheromeonfmattohiolteuhneonfe.toluene. at 673 K) is about 1.4 times as that without methanol (a maximum of 16.7% at 698 K). Moreover, the hout methanol (a maximum of 16.7% at 698 K). Moreover, the deoxygenation of xyle2n.o2l.s2C..ia2Cst.a2Calt2.yla2s.tl2t.oiyac.CtliCHayca2tnatyi.H.ao2c2tdaly.t.ryHhldCotyeiyrdtacarodiectdrHaoroeHlexydoyadytexgcidroycteorigxnoHdHoyeadengnytoediedaoxtnrtoyxnioaogydtangepieoneraondonanxdatayMydituogniMeconedtenheaMaytanphtlendia-yotd/thlMmniayoMt-aleniaxotnenhtyontdihyolfeolyMnmanlfateo-emitCtot.fih-onrmCySnelo-risaCmofeotfsimilrioemlo-nalsCv-roeColrlvyrferoe,msmePrvoste-Polh/CrZloetr/r-PovZe5eetvfs-s7/ure5oZor7lPl-t5hPot7/evtZ/reZ-r5-5P7P7t/Z-57 other reaction to produce p-/m-xylene. Similarly, the further 209 Clarteiosonlofvmer-CPrte/Zs-o5l7over Pt/Z-57 Meathteityohlnaytloiaoftinmoon-Cfomrfme-msCeo-trlCheoyrsveoleaslrotoiloPvonet/vrZeoP-rf5tP7/xZty/-Zl5e-7n5e7s also takes place which can be demonstrated by the increase of polyalkylated e which can be demonstrated by the increase of polyalkylated 2.2.12..12C..a1C2t.2a.a2Cl.t2y.a1t.lt1i.yac.Ct2liyCh.ca2tytaih.adc1tylar.yhdolCytyrditocdaeidctrohaoexhyldoydextgdrioyocerxgondhyedaeygntoedideaoxorntrxynioaoygdtangienoneoadnanxtaMadyitongiMeondetnheaMaytnahtlendiayotdthMlniayMotaelniaontehtandtdihytoaMylmnatltae-eamiCtotih-onrmCyenal-lsraCetoatstlmiri/oemM-lnsnC/-oMeCalrt/tehreMsematohsne-olaCotC/lhnMl/raoMeMnelsteoMohtlha/aoMnMarlnoaoReolrltlaMhRhtrMaiaonRntolioaollrtafioMroR1fR/ao1talfli/ato1i benzenes selectivity after adding methanol (i.e., from 2.8% without methanol to 5.3% with methanol anol (i.e., from 2.8% without methanol to 5.3% with methanol -yClarteisoonl/aMt met-hCarneosloMl/MoleatrhRanaotiloMofol1a/r1Ratio of 1/1 MyMelathteityohlnaytlaiaottinmoa-nCtamrte-msCo-lrC/eMsreoeslto/hMla/MnetoheltahMnaonlloaMlrMoRloartliaoRr aoRtfiao1ti/o1fo1f/1/1 at 673 K). ByBcyBoc-yofe-cefBoedB-yeifndyecgieocndmo-giBfB-nemfygethdecmadtiohnie-nagoftnhegelm,eoeamvdnle,aiotevnhrltigag,haorvnamuianosreoelui,mtloshvh,uemvastnrnaheimroytihloule,ayustvthlsimaoyrmtnlieiaoterthnuteihyosarylnemcmalatrtaiecieototianhioncosyntnrliloeaorasfaetnoicarosofetcinaortoeicfronaternasceanstoacatocftsnitfrotoaesrnraeosticasnrotcatoiftfenartnteimesntarsotemceordtraerimnanditntiesesatdsretoimermarsmteieamndesytdiaemaoiyrtacmeatocseyecusmdcormuicarcysatuaeyfosrso The decreased phenol and o-cresol selectivities are observed from 0.1% and 0.9% to 0.0% and 0.4% relaecttaionvntistsioeorsfiranertaecromtabnesdtesiraovtreisdnmtferaromymoecd0ci.a1utr%eassamfn(oa1dly)l0omw.(c91ecs%)tu:hmry(ta1oelsa)t0htfmio.yo0lelnl%a(o(th1towiy)foaslmnmcn:ardtoeitotf0shon.cy4ylrol%leianfstotciolrenixnsyotloflecinxnroyetolseoxnalyonilndestnmoaonxeldsytyhlmlaeyeneldaotohnmlsyisselaoatnlhnedyis[lmo3aln1ei]tsh;[o3y(y2l1lea)]n;[m3i(is21eo)t]hl;mey(2[el3a3)tht1miy]o];elna(th2toiy)folmbnaetoeintotfhzhnbeyenolnaeftztbiweoenintehzowefmnibte ieaoatnicotrnieoarnecsaticotfniorsenoascfotrafenaretcsataocntratisntotserroimnrtiendrtimeartemedseimadtieasyte(mos1cam)cymuaoyrecatochs(cuy1fcrol)ualmrltsoiaofewsonthflslo:yolflwlaoctswri:eosn:oolfinctroesxoylleintolsxyanlednmolestahnydlamniestohlyel[a3n1is]o;(le2)[3m1e]t;h(y2l)amtioenthoyflabteionnzeonfebwenitzhenm at 573 K, and from 4.1% and 3.3% to 1.1% and 1.6% at 723 K. There are several reasons for this result: 3h.1y]%l;a(na2in)sdoml1e.t[6h3%y1l]a;tt(i2o7)n2m3ofeKtbh.eyTnlzhaetinroenaworiefthbsemvntezotrehanglaeitnvroweoeaglitstoihtovolnuemgseteinofvtoelheurta[oeot3tnonh6goleiu]ilsv;[er3e(ne36ets)o]ou;[mll3u(t36:e)]tnh;emey(3[el3a)t6ht6mi]y]o;elna(t3h3toi)y)folmntaoteoeliutofhentyonoleauftpeitirononlneudopoeufrnfcoetitodnopluglurcoxeidnynulegeecpnxirnreyosglded(xnuoyec-sli,ienm(ngoe--xs,aynm(lode-n,paem-nsxd-y(opoalen-,nx,dmeyp)l-e-[xana3nyne0d)ld,e3[pnp37-e-0–x),3y3[9 mdyemlathnetyihsloyalnleains[o3isl1eo]l;[e3([213)]1;m](;2e()t2hm)yemlathteityohlnyatloiaoftinboeonfozbefenbtnoeznwgezinivetehenwetmtoiwtlehugittheimhvnaenemetot[heol3talh6una]eo;nl(oe3l)[3m6e];th(y3l)amtioenthoyflattoilounenoef tporloudeunceinpgroxdyulceinegsx(yol-e,nme-sa(nod-,pm-x-yalnednep)-x[3y0le,n3e7)– 750PDF Image | Zeolite Catalysis
PDF Search Title:
Zeolite CatalysisOriginal File Name Searched:
Zeolite_Catalysis.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |