
PDF Publication Title:
Text from PDF Page: 017
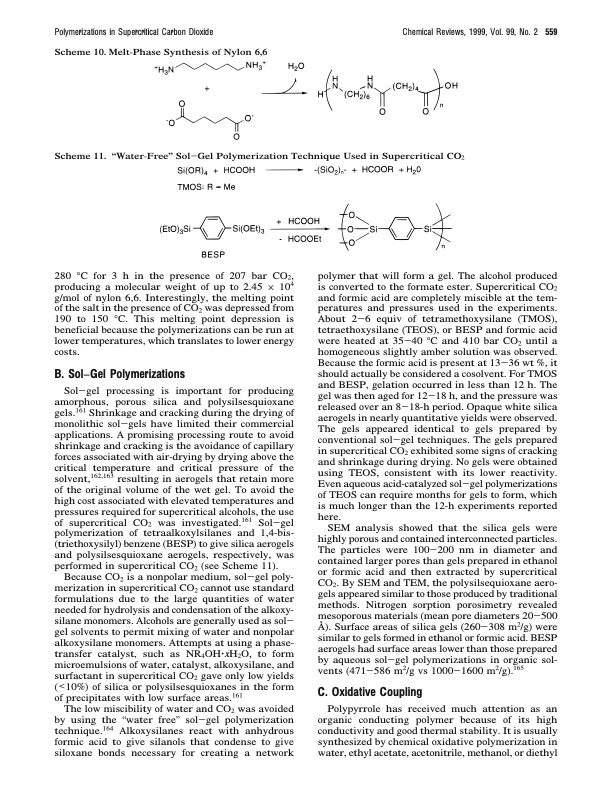
Polymerizations in Supercritical Carbon Dioxide Chemical Reviews, 1999, Vol. 99, No. 2 559 Scheme 10. Melt-Phase Synthesis of Nylon 6,6 Scheme 11. “Water-Free” Sol-Gel Polymerization Technique Used in Supercritical CO2 280 °C for 3 h in the presence of 207 bar CO2, producing a molecular weight of up to 2.45 × 104 g/mol of nylon 6,6. Interestingly, the melting point of the salt in the presence of CO2 was depressed from 190 to 150 °C. This melting point depression is beneficial because the polymerizations can be run at lower temperatures, which translates to lower energy costs. B. Sol−Gel Polymerizations Sol-gel processing is important for producing amorphous, porous silica and polysilsesquioxane gels.161 Shrinkage and cracking during the drying of monolithic sol-gels have limited their commercial applications. A promising processing route to avoid shrinkage and cracking is the avoidance of capillary forces associated with air-drying by drying above the critical temperature and critical pressure of the solvent,162,163 resulting in aerogels that retain more of the original volume of the wet gel. To avoid the high cost associated with elevated temperatures and pressures required for supercritical alcohols, the use of supercritical CO2 was investigated.161 Sol-gel polymerization of tetraalkoxylsilanes and 1,4-bis- (triethoxysilyl) benzene (BESP) to give silica aerogels and polysilsesquioxane aerogels, respectively, was performed in supercritical CO2 (see Scheme 11). Because CO2 is a nonpolar medium, sol-gel poly- merization in supercritical CO2 cannot use standard formulations due to the large quantities of water needed for hydrolysis and condensation of the alkoxy- silane monomers. Alcohols are generally used as sol- gel solvents to permit mixing of water and nonpolar alkoxysilane monomers. Attempts at using a phase- transfer catalyst, such as NR4OH‚xH2O, to form microemulsions of water, catalyst, alkoxysilane, and surfactant in supercritical CO2 gave only low yields (<10%) of silica or polysilsesquioxanes in the form of precipitates with low surface areas.161 The low miscibility of water and CO2 was avoided by using the “water free” sol-gel polymerization technique.164 Alkoxysilanes react with anhydrous formic acid to give silanols that condense to give siloxane bonds necessary for creating a network polymer that will form a gel. The alcohol produced is converted to the formate ester. Supercritical CO2 and formic acid are completely miscible at the tem- peratures and pressures used in the experiments. About 2-6 equiv of tetramethoxysilane (TMOS), tetraethoxysilane (TEOS), or BESP and formic acid were heated at 35-40 °C and 410 bar CO2 until a homogeneous slightly amber solution was observed. Because the formic acid is present at 13-36 wt %, it should actually be considered a cosolvent. For TMOS and BESP, gelation occurred in less than 12 h. The gel was then aged for 12-18 h, and the pressure was released over an 8-18-h period. Opaque white silica aerogels in nearly quantitative yields were observed. The gels appeared identical to gels prepared by conventional sol-gel techniques. The gels prepared in supercritical CO2 exhibited some signs of cracking and shrinkage during drying. No gels were obtained using TEOS, consistent with its lower reactivity. Even aqueous acid-catalyzed sol-gel polymerizations of TEOS can require months for gels to form, which is much longer than the 12-h experiments reported here. SEM analysis showed that the silica gels were highly porous and contained interconnected particles. The particles were 100-200 nm in diameter and contained larger pores than gels prepared in ethanol or formic acid and then extracted by supercritical CO2. By SEM and TEM, the polysilsequioxane aero- gels appeared similar to those produced by traditional methods. Nitrogen sorption porosimetry revealed mesoporous materials (mean pore diameters 20-500 Å). Surface areas of silica gels (260-308 m2/g) were similar to gels formed in ethanol or formic acid. BESP aerogels had surface areas lower than those prepared by aqueous sol-gel polymerizations in organic sol- vents (471-586 m2/g vs 1000-1600 m2/g).165 C. Oxidative Coupling Polypyrrole has received much attention as an organic conducting polymer because of its high conductivity and good thermal stability. It is usually synthesized by chemical oxidative polymerization in water, ethyl acetate, acetonitrile, methanol, or diethylPDF Image | Polymerizations in Supercritical Carbon Dioxide
PDF Search Title:
Polymerizations in Supercritical Carbon DioxideOriginal File Name Searched:
desimonepolymerization.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |