
PDF Publication Title:
Text from PDF Page: 018
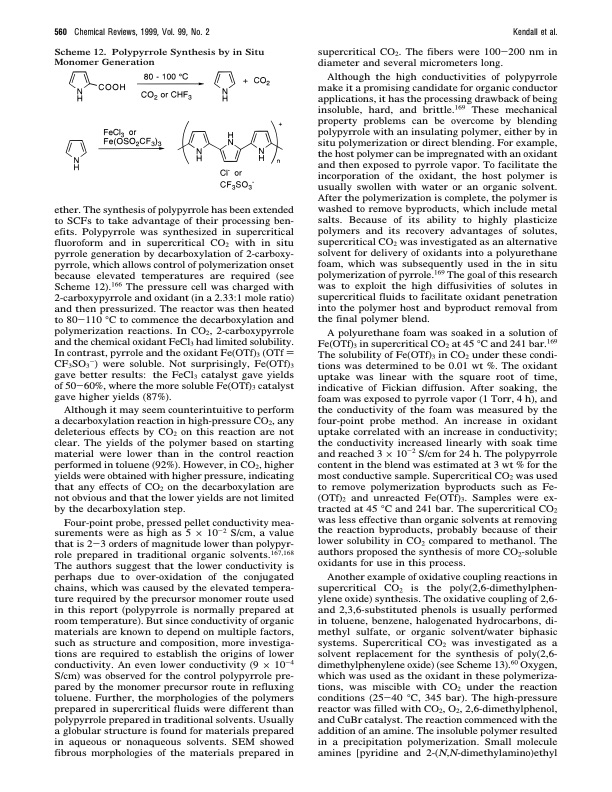
560 Chemical Reviews, 1999, Vol. 99, No. 2 Scheme 12. Polypyrrole Synthesis by in Situ Monomer Generation Kendall et al. supercritical CO2. The fibers were 100-200 nm in diameter and several micrometers long. Although the high conductivities of polypyrrole make it a promising candidate for organic conductor applications, it has the processing drawback of being insoluble, hard, and brittle.169 These mechanical property problems can be overcome by blending polypyrrole with an insulating polymer, either by in situ polymerization or direct blending. For example, the host polymer can be impregnated with an oxidant and then exposed to pyrrole vapor. To facilitate the incorporation of the oxidant, the host polymer is usually swollen with water or an organic solvent. After the polymerization is complete, the polymer is washed to remove byproducts, which include metal salts. Because of its ability to highly plasticize polymers and its recovery advantages of solutes, supercritical CO2 was investigated as an alternative solvent for delivery of oxidants into a polyurethane foam, which was subsequently used in the in situ polymerization of pyrrole.169 The goal of this research was to exploit the high diffusivities of solutes in supercritical fluids to facilitate oxidant penetration into the polymer host and byproduct removal from the final polymer blend. A polyurethane foam was soaked in a solution of Fe(OTf)3 in supercritical CO2 at 45 °C and 241 bar.169 The solubility of Fe(OTf)3 in CO2 under these condi- tions was determined to be 0.01 wt %. The oxidant uptake was linear with the square root of time, indicative of Fickian diffusion. After soaking, the foam was exposed to pyrrole vapor (1 Torr, 4 h), and the conductivity of the foam was measured by the four-point probe method. An increase in oxidant uptake correlated with an increase in conductivity; the conductivity increased linearly with soak time and reached 3 × 10-2 S/cm for 24 h. The polypyrrole content in the blend was estimated at 3 wt % for the most conductive sample. Supercritical CO2 was used to remove polymerization byproducts such as Fe- (OTf)2 and unreacted Fe(OTf)3. Samples were ex- tracted at 45 °C and 241 bar. The supercritical CO2 was less effective than organic solvents at removing the reaction byproducts, probably because of their lower solubility in CO2 compared to methanol. The authors proposed the synthesis of more CO2-soluble oxidants for use in this process. Another example of oxidative coupling reactions in supercritical CO2 is the poly(2,6-dimethylphen- ylene oxide) synthesis. The oxidative coupling of 2,6- and 2,3,6-substituted phenols is usually performed in toluene, benzene, halogenated hydrocarbons, di- methyl sulfate, or organic solvent/water biphasic systems. Supercritical CO2 was investigated as a solvent replacement for the synthesis of poly(2,6- dimethylphenylene oxide) (see Scheme 13).60 Oxygen, which was used as the oxidant in these polymeriza- tions, was miscible with CO2 under the reaction conditions (25-40 °C, 345 bar). The high-pressure reactor was filled with CO2, O2, 2,6-dimethylphenol, and CuBr catalyst. The reaction commenced with the addition of an amine. The insoluble polymer resulted in a precipitation polymerization. Small molecule amines [pyridine and 2-(N,N-dimethylamino)ethyl ether. The synthesis of polypyrrole has been extended to SCFs to take advantage of their processing ben- efits. Polypyrrole was synthesized in supercritical fluoroform and in supercritical CO2 with in situ pyrrole generation by decarboxylation of 2-carboxy- pyrrole, which allows control of polymerization onset because elevated temperatures are required (see Scheme 12).166 The pressure cell was charged with 2-carboxypyrrole and oxidant (in a 2.33:1 mole ratio) and then pressurized. The reactor was then heated to 80-110 °C to commence the decarboxylation and polymerization reactions. In CO2, 2-carboxypyrrole and the chemical oxidant FeCl3 had limited solubility. In contrast, pyrrole and the oxidant Fe(OTf)3 (OTf ) CF3SO3-) were soluble. Not surprisingly, Fe(OTf)3 gave better results: the FeCl3 catalyst gave yields of 50-60%, where the more soluble Fe(OTf)3 catalyst gave higher yields (87%). Although it may seem counterintuitive to perform a decarboxylation reaction in high-pressure CO2, any deleterious effects by CO2 on this reaction are not clear. The yields of the polymer based on starting material were lower than in the control reaction performed in toluene (92%). However, in CO2, higher yields were obtained with higher pressure, indicating that any effects of CO2 on the decarboxylation are not obvious and that the lower yields are not limited by the decarboxylation step. Four-point probe, pressed pellet conductivity mea- surements were as high as 5 × 10-2 S/cm, a value that is 2-3 orders of magnitude lower than polypyr- role prepared in traditional organic solvents.167,168 The authors suggest that the lower conductivity is perhaps due to over-oxidation of the conjugated chains, which was caused by the elevated tempera- ture required by the precursor monomer route used in this report (polypyrrole is normally prepared at room temperature). But since conductivity of organic materials are known to depend on multiple factors, such as structure and composition, more investiga- tions are required to establish the origins of lower conductivity. An even lower conductivity (9 × 10-4 S/cm) was observed for the control polypyrrole pre- pared by the monomer precursor route in refluxing toluene. Further, the morphologies of the polymers prepared in supercritical fluids were different than polypyrrole prepared in traditional solvents. Usually a globular structure is found for materials prepared in aqueous or nonaqueous solvents. SEM showed fibrous morphologies of the materials prepared inPDF Image | Polymerizations in Supercritical Carbon Dioxide
PDF Search Title:
Polymerizations in Supercritical Carbon DioxideOriginal File Name Searched:
desimonepolymerization.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |