
PDF Publication Title:
Text from PDF Page: 009
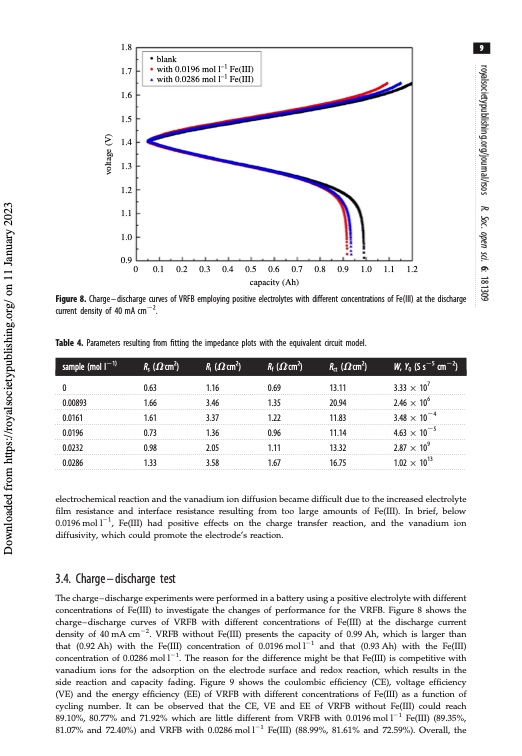
1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 9 blank with 0.0196 mol l–1 Fe(III) with 0.0286 mol l–1 Fe(III) 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2 capacity (Ah) Figure 8. Charge–discharge curves of VRFB employing positive electrolytes with different concentrations of Fe(III) at the discharge current density of 40 mA cm22. Table 4. Parameters resulting from fitting the impedance plots with the equivalent circuit model. sample (mol l21) 0 0.00893 0.0161 0.0196 0.0232 0.0286 Rs (V.cm2) 0.63 1.66 1.61 0.73 0.98 1.33 Ri (V.cm2) 1.16 3.46 3.37 1.36 2.05 3.58 Rf (V.cm2) 0.69 1.35 1.22 0.96 1.11 1.67 Rct (V.cm2) 13.11 20.94 11.83 11.14 13.32 16.75 W, Y0 (S s25 cm22) 3.33 107 2.46 106 3.48 1024 4.63 1025 2.87 109 1.02 1013 electrochemical reaction and the vanadium ion diffusion became difficult due to the increased electrolyte film resistance and interface resistance resulting from too large amounts of Fe(III). In brief, below 0.0196moll21, Fe(III) had positive effects on the charge transfer reaction, and the vanadium ion diffusivity, which could promote the electrode’s reaction. 3.4. Charge–discharge test The charge – discharge experiments were performed in a battery using a positive electrolyte with different concentrations of Fe(III) to investigate the changes of performance for the VRFB. Figure 8 shows the charge–discharge curves of VRFB with different concentrations of Fe(III) at the discharge current density of 40 mA cm22. VRFB without Fe(III) presents the capacity of 0.99 Ah, which is larger than that (0.92 Ah) with the Fe(III) concentration of 0.0196 mol l21 and that (0.93 Ah) with the Fe(III) concentration of 0.0286 mol l21. The reason for the difference might be that Fe(III) is competitive with vanadium ions for the adsorption on the electrode surface and redox reaction, which results in the side reaction and capacity fading. Figure 9 shows the coulombic efficiency (CE), voltage efficiency (VE) and the energy efficiency (EE) of VRFB with different concentrations of Fe(III) as a function of cycling number. It can be observed that the CE, VE and EE of VRFB without Fe(III) could reach 89.10%, 80.77% and 71.92% which are little different from VRFB with 0.0196 mol l21 Fe(III) (89.35%, 81.07% and 72.40%) and VRFB with 0.0286 mol l21 Fe(III) (88.99%, 81.61% and 72.59%). Overall, the royalsocietypublishing.org/journal/rsos R. Soc. open sci. 6: 181309 Downloaded from https://royalsocietypublishing.org/ on 11 January 2023 voltage (V)PDF Image | Effect of Fe3 positive electrolyte vanadium redox flow
PDF Search Title:
Effect of Fe3 positive electrolyte vanadium redox flowOriginal File Name Searched:
rsos-181309.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |