
PDF Publication Title:
Text from PDF Page: 010
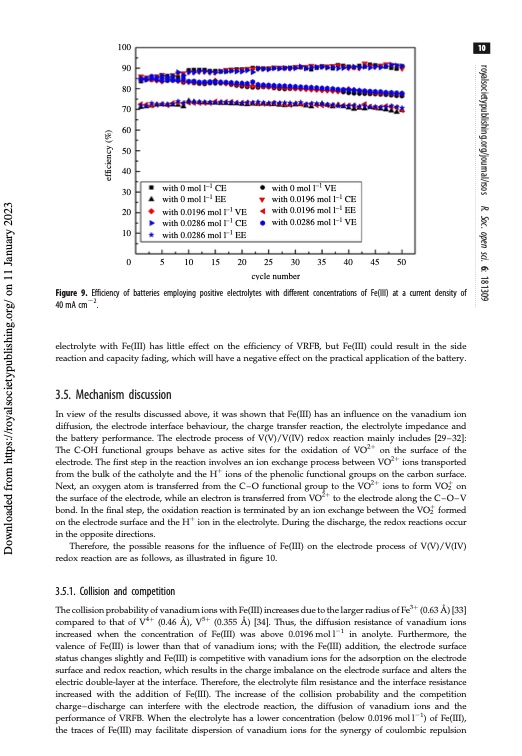
100 90 80 70 60 50 40 30 20 10 0 10 with 0 mol l–1 CE with 0 mol l–1 EE with 0.0196 mol l–1 VE with 0.0286 mol l–1 CE with 0.0286 mol l–1 EE with 0 mol l–1 VE with 0.0196 mol l–1 CE with 0.0196 mol l–1 EE with 0.0286 mol l–1 VE 5 10 15 20 25 30 35 40 45 50 cycle number Figure 9. Efficiency of batteries employing positive electrolytes with different concentrations of Fe(III) at a current density of 40 mA cm22. electrolyte with Fe(III) has little effect on the efficiency of VRFB, but Fe(III) could result in the side reaction and capacity fading, which will have a negative effect on the practical application of the battery. 3.5. Mechanism discussion In view of the results discussed above, it was shown that Fe(III) has an influence on the vanadium ion diffusion, the electrode interface behaviour, the charge transfer reaction, the electrolyte impedance and the battery performance. The electrode process of V(V)/V(IV) redox reaction mainly includes [29–32]: The C-OH functional groups behave as active sites for the oxidation of VO2þ on the surface of the electrode. The first step in the reaction involves an ion exchange process between VO2þ ions transported from the bulk of the catholyte and the Hþ ions of the phenolic functional groups on the carbon surface. Next, an oxygen atom is transferred from the C–O functional group to the VO2þ ions to form VOþ2 on the surface of the electrode, while an electron is transferred from VO2þ to the electrode along the C–O–V bond. In the final step, the oxidation reaction is terminated by an ion exchange between the VOþ2 formed on the electrode surface and the Hþ ion in the electrolyte. During the discharge, the redox reactions occur in the opposite directions. Therefore, the possible reasons for the influence of Fe(III) on the electrode process of V(V)/V(IV) redox reaction are as follows, as illustrated in figure 10. 3.5.1. Collision and competition The collision probability of vanadium ions with Fe(III) increases due to the larger radius of Fe3þ (0.63 A ̊ ) [33] compared to that of V4þ (0.46 A ̊ ), V5þ (0.355 A ̊ ) [34]. Thus, the diffusion resistance of vanadium ions increased when the concentration of Fe(III) was above 0.0196moll21 in anolyte. Furthermore, the valence of Fe(III) is lower than that of vanadium ions; with the Fe(III) addition, the electrode surface status changes slightly and Fe(III) is competitive with vanadium ions for the adsorption on the electrode surface and redox reaction, which results in the charge imbalance on the electrode surface and alters the electric double-layer at the interface. Therefore, the electrolyte film resistance and the interface resistance increased with the addition of Fe(III). The increase of the collision probability and the competition charge–discharge can interfere with the electrode reaction, the diffusion of vanadium ions and the performance of VRFB. When the electrolyte has a lower concentration (below 0.0196 mol l21) of Fe(III), the traces of Fe(III) may facilitate dispersion of vanadium ions for the synergy of coulombic repulsion royalsocietypublishing.org/journal/rsos R. Soc. open sci. 6: 181309 Downloaded from https://royalsocietypublishing.org/ on 11 January 2023 efficiency (%)PDF Image | Effect of Fe3 positive electrolyte vanadium redox flow
PDF Search Title:
Effect of Fe3 positive electrolyte vanadium redox flowOriginal File Name Searched:
rsos-181309.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |