
PDF Publication Title:
Text from PDF Page: 002
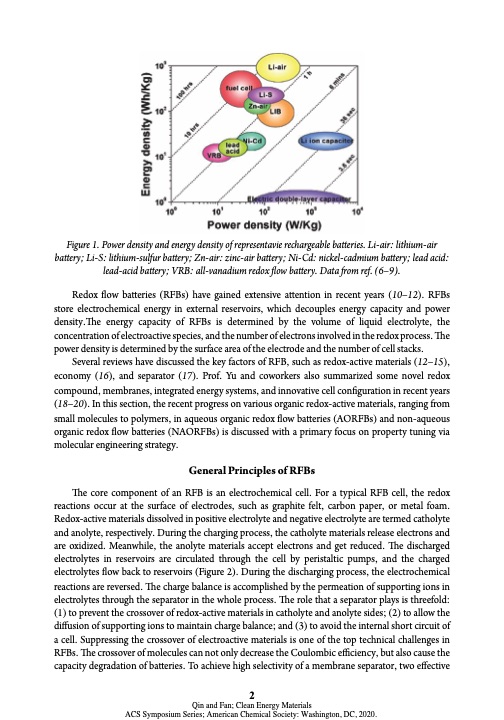
Figure 1. Power density and energy density of representavie rechargeable batteries. Li-air: lithium-air battery; Li-S: lithium-sulfur battery; Zn-air: zinc-air battery; Ni-Cd: nickel-cadmium battery; lead acid: lead-acid battery; VRB: all-vanadium redox flow battery. Data from ref. (6–9). Redox flow batteries (RFBs) have gained extensive attention in recent years (10–12). RFBs store electrochemical energy in external reservoirs, which decouples energy capacity and power density.The energy capacity of RFBs is determined by the volume of liquid electrolyte, the concentration of electroactive species, and the number of electrons involved in the redox process. The power density is determined by the surface area of the electrode and the number of cell stacks. Several reviews have discussed the key factors of RFB, such as redox-active materials (12–15), economy (16), and separator (17). Prof. Yu and coworkers also summarized some novel redox compound, membranes, integrated energy systems, and innovative cell configuration in recent years (18–20). In this section, the recent progress on various organic redox-active materials, ranging from small molecules to polymers, in aqueous organic redox flow batteries (AORFBs) and non-aqueous organic redox flow batteries (NAORFBs) is discussed with a primary focus on property tuning via molecular engineering strategy. General Principles of RFBs The core component of an RFB is an electrochemical cell. For a typical RFB cell, the redox reactions occur at the surface of electrodes, such as graphite felt, carbon paper, or metal foam. Redox-active materials dissolved in positive electrolyte and negative electrolyte are termed catholyte and anolyte, respectively. During the charging process, the catholyte materials release electrons and are oxidized. Meanwhile, the anolyte materials accept electrons and get reduced. The discharged electrolytes in reservoirs are circulated through the cell by peristaltic pumps, and the charged electrolytes flow back to reservoirs (Figure 2). During the discharging process, the electrochemical reactions are reversed. The charge balance is accomplished by the permeation of supporting ions in electrolytes through the separator in the whole process. The role that a separator plays is threefold: (1) to prevent the crossover of redox-active materials in catholyte and anolyte sides; (2) to allow the diffusion of supporting ions to maintain charge balance; and (3) to avoid the internal short circuit of a cell. Suppressing the crossover of electroactive materials is one of the top technical challenges in RFBs. The crossover of molecules can not only decrease the Coulombic efficiency, but also cause the capacity degradation of batteries. To achieve high selectivity of a membrane separator, two effective 2 Qin and Fan; Clean Energy Materials ACS Symposium Series; American Chemical Society: Washington, DC, 2020.PDF Image | Electroactive Materials Next-Generation Redox Flow Batteries
PDF Search Title:
Electroactive Materials Next-Generation Redox Flow BatteriesOriginal File Name Searched:
bk-2020-1364.ch001.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |