
PDF Publication Title:
Text from PDF Page: 031
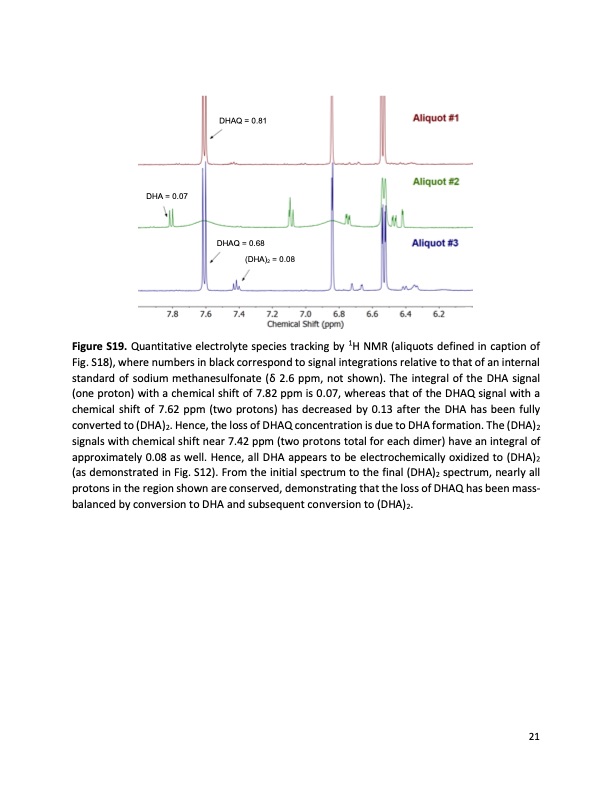
DHAQ = 0.81 DHA = 0.07 Figure S19. DHAQ = 0.68 (DHA)2 = 0.08 Quantitative electrolyte species tracking by 1H NMR (aliquots defined in caption of Fig. S18), where numbers in black correspond to signal integrations relative to that of an internal standard of sodium methanesulfonate ( δ 2.6 ppm, not shown ). The integral of the DHA signal (one proton) with a chemical shift of 7.82 ppm is 0.07, whereas that of the DHAQ signal with a chemical shift of 7.62 ppm (two protons) has decreased by 0.13 after the DHA has been fully converted to (DHA)2. Hence, the loss of DHAQ concentration is due to DHA formation. The (DHA)2 signals with chemical shift near 7.42 ppm (two protons total for each dimer) have an integral of approximately 0.08 as well. Hence, all DHA appears to be electrochemically oxidized to (DHA)2 (as demonstrated in Fig. S12). From the initial spectrum to the final (DHA)2 spectrum, nearly all protons in the region shown are conserved, demonstrating that the loss of DHAQ has been mass- balanced by conversion to DHA and subsequent conversion to (DHA)2. 21PDF Image | Extending organic flow batteries via redox state management
PDF Search Title:
Extending organic flow batteries via redox state managementOriginal File Name Searched:
mja287.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |