
PDF Publication Title:
Text from PDF Page: 001
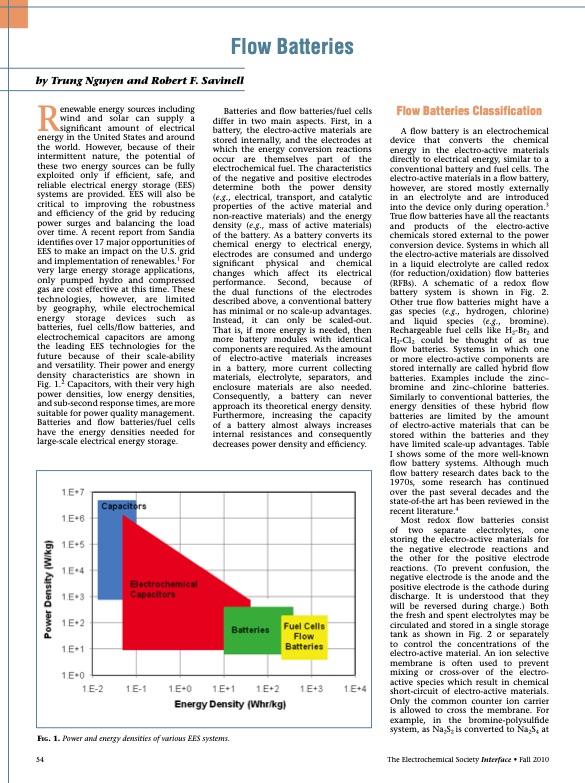
Flow Batteries by Trung Nguyen and Robert F. Savinell Renewable energy sources including wind and solar can supply a significant amount of electrical energy in the United States and around the world. However, because of their intermittent nature, the potential of these two energy sources can be fully exploited only if efficient, safe, and reliable electrical energy storage (EES) systems are provided. EES will also be critical to improving the robustness and efficiency of the grid by reducing power surges and balancing the load over time. A recent report from Sandia identifies over 17 major opportunities of EES to make an impact on the U.S. grid and implementation of renewables.1 For very large energy storage applications, only pumped hydro and compressed gas are cost effective at this time. These technologies, however, are limited by geography, while electrochemical energy storage devices such as batteries, fuel cells/flow batteries, and electrochemical capacitors are among the leading EES technologies for the future because of their scale-ability and versatility. Their power and energy density characteristics are shown in Fig. 1.2 Capacitors, with their very high power densities, low energy densities, and sub-second response times, are more suitable for power quality management. Batteries and flow batteries/fuel cells have the energy densities needed for large-scale electrical energy storage. Batteries and flow batteries/fuel cells differ in two main aspects. First, in a battery, the electro-active materials are stored internally, and the electrodes at which the energy conversion reactions occur are themselves part of the electrochemical fuel. The characteristics of the negative and positive electrodes determine both the power density (e.g., electrical, transport, and catalytic properties of the active material and non-reactive materials) and the energy density (e.g., mass of active materials) of the battery. As a battery converts its chemical energy to electrical energy, electrodes are consumed and undergo significant physical and chemical changes which affect its electrical performance. Second, because of the dual functions of the electrodes described above, a conventional battery has minimal or no scale-up advantages. Instead, it can only be scaled-out. That is, if more energy is needed, then more battery modules with identical components are required. As the amount of electro-active materials increases in a battery, more current collecting materials, electrolyte, separators, and enclosure materials are also needed. Consequently, a battery can never approach its theoretical energy density. Furthermore, increasing the capacity of a battery almost always increases internal resistances and consequently decreases power density and efficiency. Flow Batteries Classification A flow battery is an electrochemical device that converts the chemical energy in the electro-active materials directly to electrical energy, similar to a conventional battery and fuel cells. The electro-active materials in a flow battery, however, are stored mostly externally in an electrolyte and are introduced into the device only during operation.3 True flow batteries have all the reactants and products of the electro-active chemicals stored external to the power conversion device. Systems in which all the electro-active materials are dissolved in a liquid electrolyte are called redox (for reduction/oxidation) flow batteries (RFBs). A schematic of a redox flow battery system is shown in Fig. 2. Other true flow batteries might have a gas species (e.g., hydrogen, chlorine) and liquid species (e.g., bromine). Rechargeable fuel cells like H2-Br2 and H2-Cl2 could be thought of as true flow batteries. Systems in which one or more electro-active components are stored internally are called hybrid flow batteries. Examples include the zinc– bromine and zinc–chlorine batteries. Similarly to conventional batteries, the energy densities of these hybrid flow batteries are limited by the amount of electro-active materials that can be stored within the batteries and they have limited scale-up advantages. Table I shows some of the more well-known flow battery systems. Although much flow battery research dates back to the 1970s, some research has continued over the past several decades and the state-of-the art has been reviewed in the recent literature.4 Most redox flow batteries consist of two separate electrolytes, one storing the electro-active materials for the negative electrode reactions and the other for the positive electrode reactions. (To prevent confusion, the negative electrode is the anode and the positive electrode is the cathode during discharge. It is understood that they will be reversed during charge.) Both the fresh and spent electrolytes may be circulated and stored in a single storage tank as shown in Fig. 2 or separately to control the concentrations of the electro-active material. An ion selective membrane is often used to prevent mixing or cross-over of the electro- active species which result in chemical short-circuit of electro-active materials. Only the common counter ion carrier is allowed to cross the membrane. For example, in the bromine-polysulfide system, as Na2S2 is converted to Na2S4 at The Electrochemical Society Interface • Fall 2010 Fig. 1. Power and energy densities of various EES systems. 54PDF Image | Flow Batteries 2010
PDF Search Title:
Flow Batteries 2010Original File Name Searched:
fal10_p054-056.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |