
PDF Publication Title:
Text from PDF Page: 026
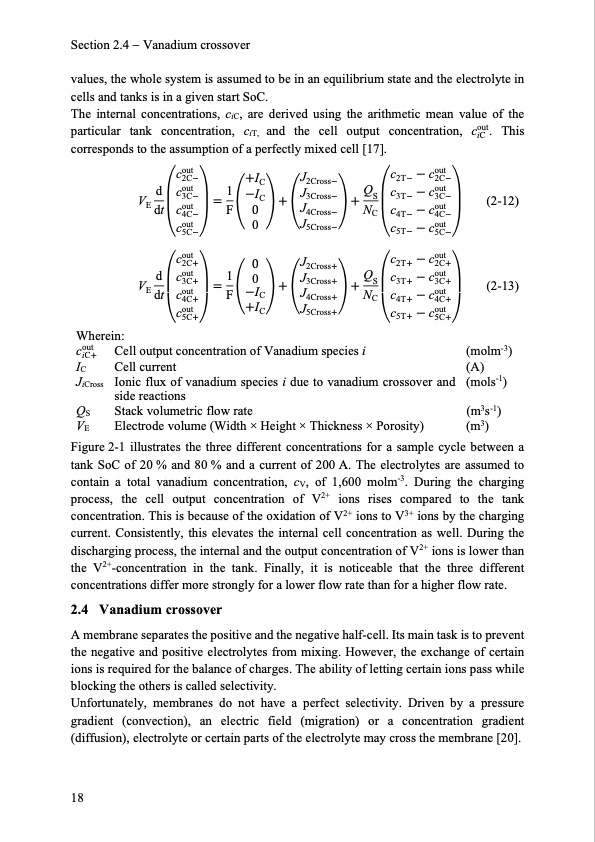
Section 2.4 Vanadium crossover values, the whole system is assumed to be in an equilibrium state and the electrolyte in cells and tanks is in a given start SoC. The internal concentrations, ciC, are derived using the arithmetic mean value of the particular tank concentration, ciT, and the cell output concentration, cout. This iC corresponds to the assumption of a perfectly mixed cell [17]. cout 2C IC dcout 1I V 3C C 3Cross S 3C (2-12) E dt 0 J4Cross 0 J5Cross Qc3Tcout cout 4C F N C c cout 4T 4C cout 5C cout 2C 0 J2Cross c cout 5T 5C c cout 2T 2C J2Cross J c cout 2T 2C dcout 1 0 J V 3C I 3Cross S 3C (2-13) E dt cout F C 4C J4Cross N J5Cross C c cout 4T 4C cout IC 5C c cout 5T 5C Wherein: cout Cell output concentration of Vanadium species i (molm-3) (A) (mols-1) (m3s-1) (m3) iC IC Cell current JiCross Ionic flux of vanadium species i due to vanadium crossover and side reactions QS Stack volumetric flow rate VE Electrode volume (Width × Height × Thickness × Porosity) Figure 2-1 illustrates the three different concentrations for a sample cycle between a tank SoC of 20 % and 80 % and a current of 200 A. The electrolytes are assumed to contain a total vanadium concentration, cV, of 1,600 molm-3. During the charging process, the cell output concentration of V2+ ions rises compared to the tank concentration. This is because of the oxidation of V2+ ions to V3+ ions by the charging current. Consistently, this elevates the internal cell concentration as well. During the discharging process, the internal and the output concentration of V2+ ions is lower than the V2+-concentration in the tank. Finally, it is noticeable that the three different concentrations differ more strongly for a lower flow rate than for a higher flow rate. 2.4 Vanadium crossover A membrane separates the positive and the negative half-cell. Its main task is to prevent the negative and positive electrolytes from mixing. However, the exchange of certain ions is required for the balance of charges. The ability of letting certain ions pass while blocking the others is called selectivity. Unfortunately, membranes do not have a perfect selectivity. Driven by a pressure gradient (convection), an electric field (migration) or a concentration gradient (diffusion), electrolyte or certain parts of the electrolyte may cross the membrane [20]. 18 Q c3Tcout PDF Image | Model-based Design Vanadium Redox Flow Batteries
PDF Search Title:
Model-based Design Vanadium Redox Flow BatteriesOriginal File Name Searched:
10-5445IR1000070670.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |