
PDF Publication Title:
Text from PDF Page: 129
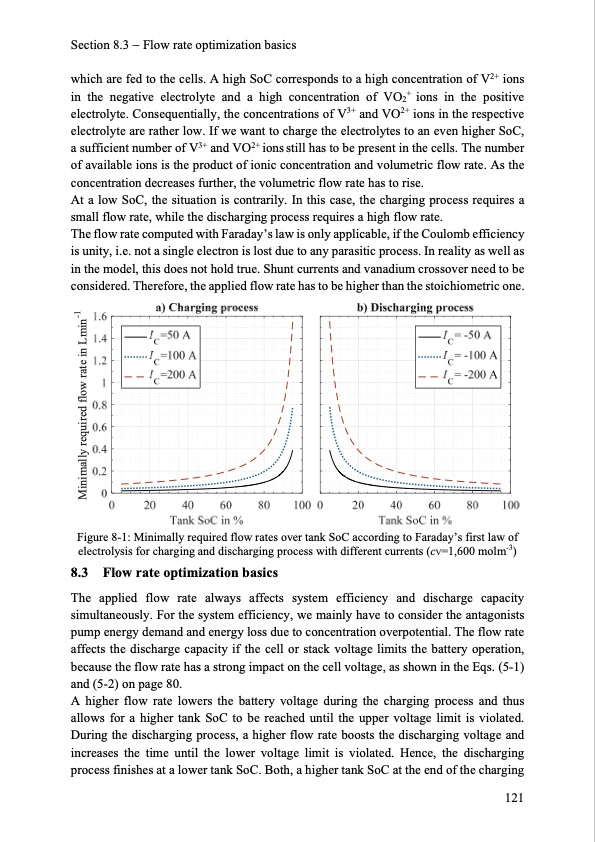
Section 8.3 Flow rate optimization basics which are fed to the cells. A high SoC corresponds to a high concentration of V2+ ions in the negative electrolyte and a high concentration of VO2+ ions in the positive electrolyte. Consequentially, the concentrations of V3+ and VO2+ ions in the respective electrolyte are rather low. If we want to charge the electrolytes to an even higher SoC, a sufficient number of V3+ and VO2+ ions still has to be present in the cells. The number of available ions is the product of ionic concentration and volumetric flow rate. As the concentration decreases further, the volumetric flow rate has to rise. At a low SoC, the situation is contrarily. In this case, the charging process requires a small flow rate, while the discharging process requires a high flow rate. The flow rate computed with Faraday’s law is only applicable, if the Coulomb efficiency is unity, i.e. not a single electron is lost due to any parasitic process. In reality as well as in the model, this does not hold true. Shunt currents and vanadium crossover need to be considered. Therefore, the applied flow rate has to be higher than the stoichiometric one. Figure 8-1: Minimally required flow rates over tank SoC according to Faraday’s first law of electrolysis for charging and discharging process with different currents (cV=1,600 molm-3) 8.3 Flow rate optimization basics The applied flow rate always affects system efficiency and discharge capacity simultaneously. For the system efficiency, we mainly have to consider the antagonists pump energy demand and energy loss due to concentration overpotential. The flow rate affects the discharge capacity if the cell or stack voltage limits the battery operation, because the flow rate has a strong impact on the cell voltage, as shown in the Eqs. (5-1) and (5-2) on page 80. A higher flow rate lowers the battery voltage during the charging process and thus allows for a higher tank SoC to be reached until the upper voltage limit is violated. During the discharging process, a higher flow rate boosts the discharging voltage and increases the time until the lower voltage limit is violated. Hence, the discharging process finishes at a lower tank SoC. Both, a higher tank SoC at the end of the charging 121 Minimally required flow rate in Lmin-1PDF Image | Model-based Design Vanadium Redox Flow Batteries
PDF Search Title:
Model-based Design Vanadium Redox Flow BatteriesOriginal File Name Searched:
10-5445IR1000070670.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |