
PDF Publication Title:
Text from PDF Page: 033
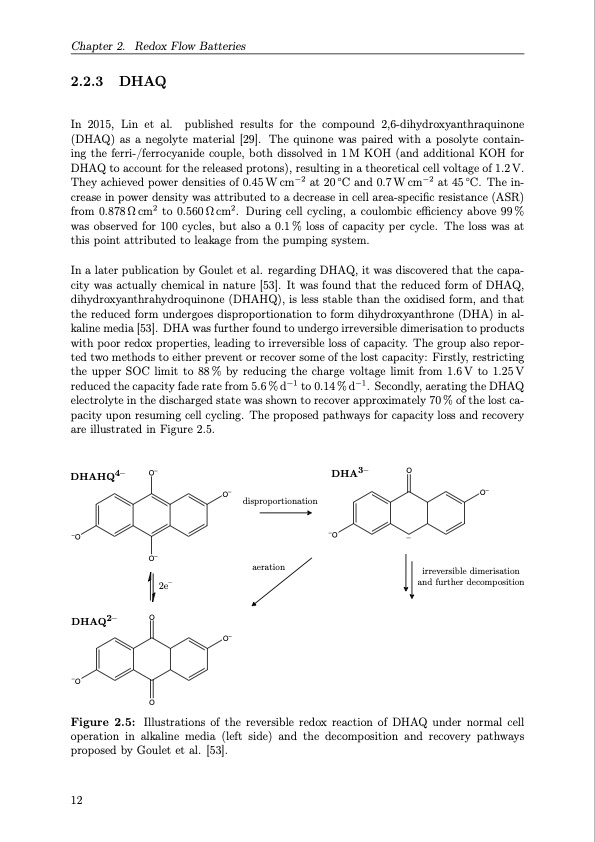
Chapter 2. Redox Flow Batteries 2.2.3 DHAQ In 2015, Lin et al. published results for the compound 2,6-dihydroxyanthraquinone (DHAQ) as a negolyte material [29]. The quinone was paired with a posolyte contain- ing the ferri-/ferrocyanide couple, both dissolved in 1M KOH (and additional KOH for DHAQ to account for the released protons), resulting in a theoretical cell voltage of 1.2 V. They achieved power densities of 0.45 W cm−2 at 20 ◦C and 0.7 W cm−2 at 45 ◦C. The in- crease in power density was attributed to a decrease in cell area-specific resistance (ASR) from 0.878Ωcm2 to 0.560Ωcm2. During cell cycling, a coulombic efficiency above 99% was observed for 100 cycles, but also a 0.1 % loss of capacity per cycle. The loss was at this point attributed to leakage from the pumping system. In a later publication by Goulet et al. regarding DHAQ, it was discovered that the capa- city was actually chemical in nature [53]. It was found that the reduced form of DHAQ, dihydroxyanthrahydroquinone (DHAHQ), is less stable than the oxidised form, and that the reduced form undergoes disproportionation to form dihydroxyanthrone (DHA) in al- kaline media [53]. DHA was further found to undergo irreversible dimerisation to products with poor redox properties, leading to irreversible loss of capacity. The group also repor- ted two methods to either prevent or recover some of the lost capacity: Firstly, restricting the upper SOC limit to 88% by reducing the charge voltage limit from 1.6V to 1.25V reduced the capacity fade rate from 5.6 % d−1 to 0.14 % d−1. Secondly, aerating the DHAQ electrolyte in the discharged state was shown to recover approximately 70 % of the lost ca- pacity upon resuming cell cycling. The proposed pathways for capacity loss and recovery are illustrated in Figure 2.5. DHA3– O –O –O– DHAHQ4– O– O– disproportionation O– irreversible dimerisation and further decomposition DHAQ2– –O O– O– O O aeration 2e– Figure 2.5: Illustrations of the reversible redox reaction of DHAQ under normal cell operation in alkaline media (left side) and the decomposition and recovery pathways proposed by Goulet et al. [53]. 12PDF Image | Organic Redox Flow Batteries 2023
PDF Search Title:
Organic Redox Flow Batteries 2023Original File Name Searched:
PhD_thesis_final_dorhoff_4_.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |