
PDF Publication Title:
Text from PDF Page: 036
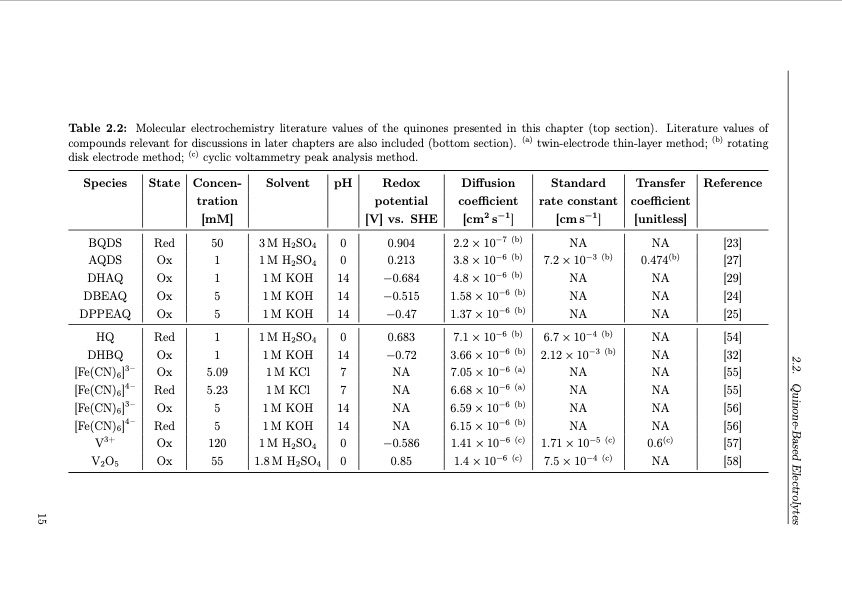
2.2. Quinone-Based Electrolytes 15 Table 2.2: Molecular electrochemistry literature values of the quinones presented in this chapter (top section). Literature values of compounds relevant for discussions in later chapters are also included (bottom section). (a) twin-electrode thin-layer method; (b) rotating disk electrode method; (c) cyclic voltammetry peak analysis method. Species State Concen- tration Solvent pH Redox potential Diffusion coefficient [cm2 s−1] Standard rate constant [cm s−1] Transfer Reference coefficient BQDS Red 50 AQDS Ox 1 DHAQ Ox 1 DBEAQ Ox 5 DPPEAQ Ox 5 3MH2SO4 1MH2SO4 1MKOH 1MKOH 1MKOH 0 0.904 2.2×10−7 (b) 3.8×10−6 (b) NA NA [23] 0.474(b) [27] NA [29] NA [24] NA [25] HQ Red 1 Ox 1 Ox 5.09 1M H2SO4 1MKOH 1M KCl 1M KCl 1M KOH 1M KOH 1M H2SO4 1.8M H2SO4 0 0.683 14 −0.72 7 NA 7.1×10−6 (b) 6.7×10−4 (b) 2.12×10−3 (b) NA NA NA NA 1.71×10−5 (c) 7.5×10−4 (c) NA [54] NA [32] NA [55] NA [55] NA [56] NA [56] DHBQ 3.66 × 10−6 (b) [Fe(CN)6]3– 7.05 × 10−6 (a) [Fe(CN)6]4– Red 5.23 Ox 5 Red 5 7 NA 14 NA 14 NA 6.68 × 10−6 (a) [Fe(CN)6]3– 6.59 × 10−6 (b) [Fe(CN)6]4– V3+ Ox 120 Ox 55 0 −0.586 0 0.85 6.15 × 10−6 (b) 0.6(c) [57] NA [58] V2O5 1.41 × 10−6 (c) 1.4×10−6 (c) [mM] [V] vs. SHE [unitless] 0 0.213 14 −0.684 14 −0.515 14 −0.47 4.8×10−6 (b) 1.58 × 10−6 (b) 7.2×10−3 (b) NA NA NA 1.37 × 10−6 (b)PDF Image | Organic Redox Flow Batteries 2023
PDF Search Title:
Organic Redox Flow Batteries 2023Original File Name Searched:
PhD_thesis_final_dorhoff_4_.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |