
PDF Publication Title:
Text from PDF Page: 075
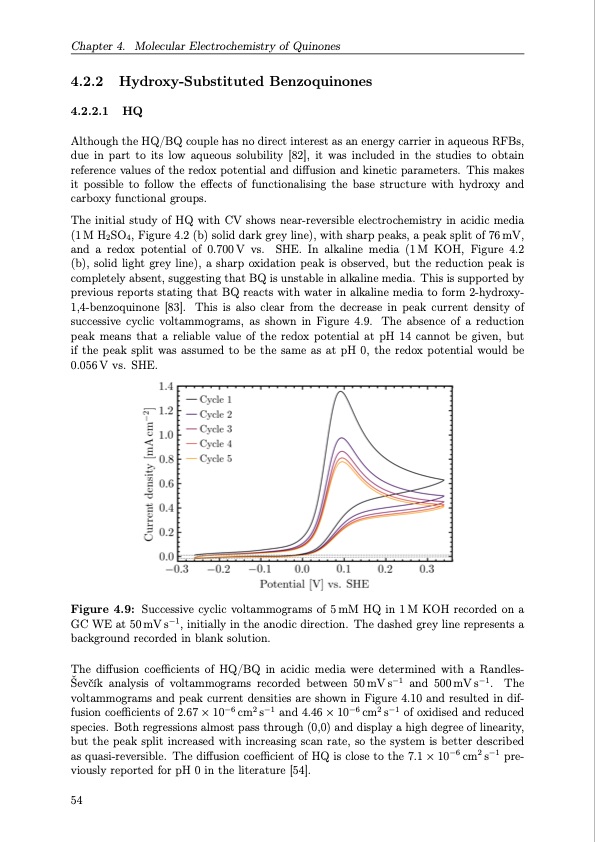
Chapter 4. Molecular Electrochemistry of Quinones 4.2.2 Hydroxy-Substituted Benzoquinones 4.2.2.1 HQ Although the HQ/BQ couple has no direct interest as an energy carrier in aqueous RFBs, due in part to its low aqueous solubility [82], it was included in the studies to obtain reference values of the redox potential and diffusion and kinetic parameters. This makes it possible to follow the effects of functionalising the base structure with hydroxy and carboxy functional groups. The initial study of HQ with CV shows near-reversible electrochemistry in acidic media (1 M H2SO4, Figure 4.2 (b) solid dark grey line), with sharp peaks, a peak split of 76 mV, and a redox potential of 0.700V vs. SHE. In alkaline media (1M KOH, Figure 4.2 (b), solid light grey line), a sharp oxidation peak is observed, but the reduction peak is completely absent, suggesting that BQ is unstable in alkaline media. This is supported by previous reports stating that BQ reacts with water in alkaline media to form 2-hydroxy- 1,4-benzoquinone [83]. This is also clear from the decrease in peak current density of successive cyclic voltammograms, as shown in Figure 4.9. The absence of a reduction peak means that a reliable value of the redox potential at pH 14 cannot be given, but if the peak split was assumed to be the same as at pH 0, the redox potential would be 0.056 V vs. SHE. Figure 4.9: Successive cyclic voltammograms of 5 mM HQ in 1 M KOH recorded on a GC WE at 50 mV s−1, initially in the anodic direction. The dashed grey line represents a background recorded in blank solution. The diffusion coefficients of HQ/BQ in acidic media were determined with a Randles- Ševčík analysis of voltammograms recorded between 50 mV s−1 and 500 mV s−1. The voltammograms and peak current densities are shown in Figure 4.10 and resulted in dif- fusion coefficients of 2.67 × 10−6 cm2 s−1 and 4.46 × 10−6 cm2 s−1 of oxidised and reduced species. Both regressions almost pass through (0,0) and display a high degree of linearity, but the peak split increased with increasing scan rate, so the system is better described as quasi-reversible. The diffusion coefficient of HQ is close to the 7.1 × 10−6 cm2 s−1 pre- viously reported for pH 0 in the literature [54]. 54PDF Image | Organic Redox Flow Batteries 2023
PDF Search Title:
Organic Redox Flow Batteries 2023Original File Name Searched:
PhD_thesis_final_dorhoff_4_.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |