
PDF Publication Title:
Text from PDF Page: 076
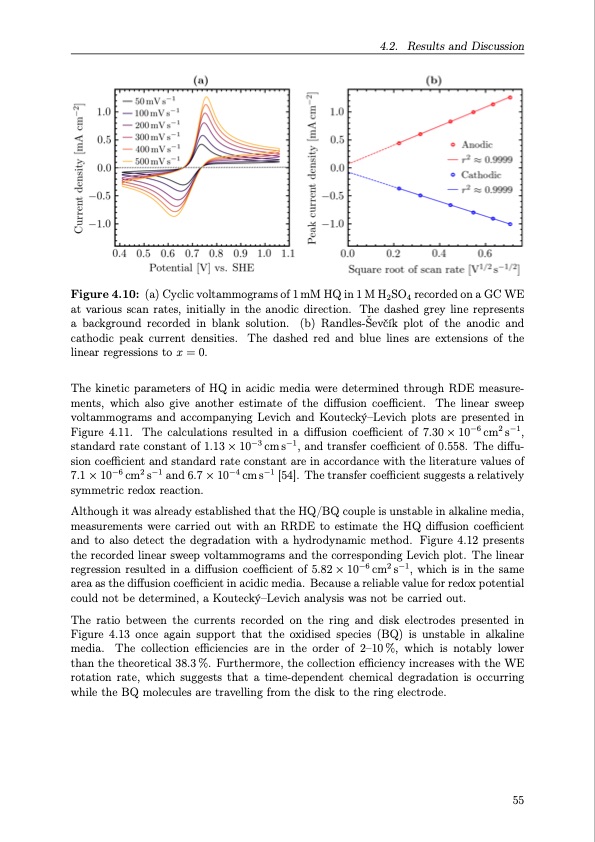
4.2. Results and Discussion Figure 4.10: (a) Cyclic voltammograms of 1 mM HQ in 1 M H2SO4 recorded on a GC WE at various scan rates, initially in the anodic direction. The dashed grey line represents a background recorded in blank solution. (b) Randles-Ševčík plot of the anodic and cathodic peak current densities. The dashed red and blue lines are extensions of the linear regressions to x = 0. The kinetic parameters of HQ in acidic media were determined through RDE measure- ments, which also give another estimate of the diffusion coefficient. The linear sweep voltammograms and accompanying Levich and Koutecký–Levich plots are presented in Figure 4.11. The calculations resulted in a diffusion coefficient of 7.30 × 10−6 cm2 s−1, standard rate constant of 1.13 × 10−3 cm s−1, and transfer coefficient of 0.558. The diffu- sion coefficient and standard rate constant are in accordance with the literature values of 7.1 × 10−6 cm2 s−1 and 6.7 × 10−4 cm s−1 [54]. The transfer coefficient suggests a relatively symmetric redox reaction. Although it was already established that the HQ/BQ couple is unstable in alkaline media, measurements were carried out with an RRDE to estimate the HQ diffusion coefficient and to also detect the degradation with a hydrodynamic method. Figure 4.12 presents the recorded linear sweep voltammograms and the corresponding Levich plot. The linear regression resulted in a diffusion coefficient of 5.82 × 10−6 cm2 s−1, which is in the same area as the diffusion coefficient in acidic media. Because a reliable value for redox potential could not be determined, a Koutecký–Levich analysis was not be carried out. The ratio between the currents recorded on the ring and disk electrodes presented in Figure 4.13 once again support that the oxidised species (BQ) is unstable in alkaline media. The collection efficiencies are in the order of 2–10%, which is notably lower than the theoretical 38.3 %. Furthermore, the collection efficiency increases with the WE rotation rate, which suggests that a time-dependent chemical degradation is occurring while the BQ molecules are travelling from the disk to the ring electrode. 55PDF Image | Organic Redox Flow Batteries 2023
PDF Search Title:
Organic Redox Flow Batteries 2023Original File Name Searched:
PhD_thesis_final_dorhoff_4_.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |