
PDF Publication Title:
Text from PDF Page: 127
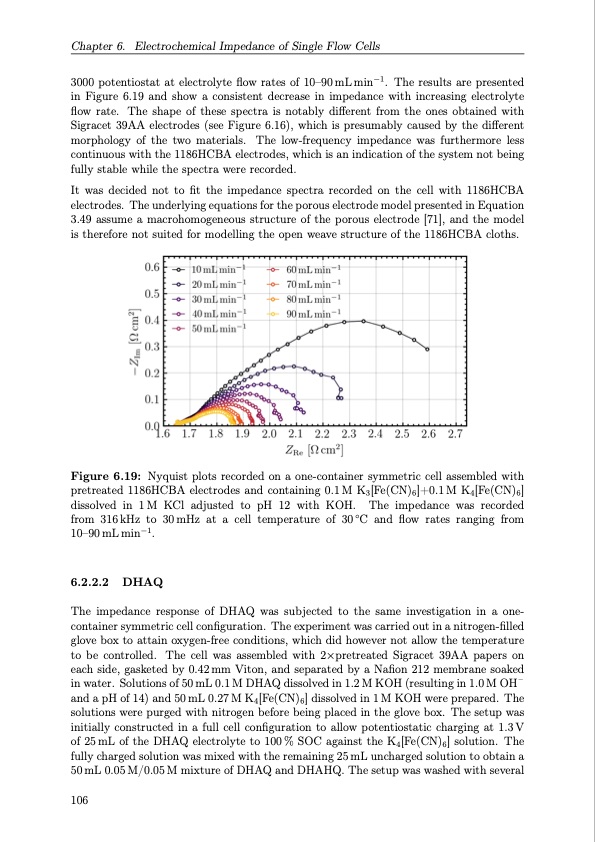
Chapter 6. Electrochemical Impedance of Single Flow Cells 3000 potentiostat at electrolyte flow rates of 10–90 mL min−1. The results are presented in Figure 6.19 and show a consistent decrease in impedance with increasing electrolyte flow rate. The shape of these spectra is notably different from the ones obtained with Sigracet 39AA electrodes (see Figure 6.16), which is presumably caused by the different morphology of the two materials. The low-frequency impedance was furthermore less continuous with the 1186HCBA electrodes, which is an indication of the system not being fully stable while the spectra were recorded. It was decided not to fit the impedance spectra recorded on the cell with 1186HCBA electrodes. The underlying equations for the porous electrode model presented in Equation 3.49 assume a macrohomogeneous structure of the porous electrode [71], and the model is therefore not suited for modelling the open weave structure of the 1186HCBA cloths. Figure 6.19: Nyquist plots recorded on a one-container symmetric cell assembled with pretreated 1186HCBA electrodes and containing 0.1 M K3[Fe(CN)6]+0.1 M K4[Fe(CN)6] dissolved in 1M KCl adjusted to pH 12 with KOH. The impedance was recorded from 316kHz to 30mHz at a cell temperature of 30◦C and flow rates ranging from 10–90 mL min−1. 6.2.2.2 DHAQ The impedance response of DHAQ was subjected to the same investigation in a one- container symmetric cell configuration. The experiment was carried out in a nitrogen-filled glove box to attain oxygen-free conditions, which did however not allow the temperature to be controlled. The cell was assembled with 2×pretreated Sigracet 39AA papers on each side, gasketed by 0.42 mm Viton, and separated by a Nafion 212 membrane soaked in water. Solutions of 50 mL 0.1 M DHAQ dissolved in 1.2 M KOH (resulting in 1.0 M OH – and a pH of 14) and 50 mL 0.27 M K4[Fe(CN)6] dissolved in 1 M KOH were prepared. The solutions were purged with nitrogen before being placed in the glove box. The setup was initially constructed in a full cell configuration to allow potentiostatic charging at 1.3V of 25mL of the DHAQ electrolyte to 100% SOC against the K4[Fe(CN)6] solution. The fully charged solution was mixed with the remaining 25 mL uncharged solution to obtain a 50 mL 0.05 M/0.05 M mixture of DHAQ and DHAHQ. The setup was washed with several 106PDF Image | Organic Redox Flow Batteries 2023
PDF Search Title:
Organic Redox Flow Batteries 2023Original File Name Searched:
PhD_thesis_final_dorhoff_4_.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |