
PDF Publication Title:
Text from PDF Page: 003
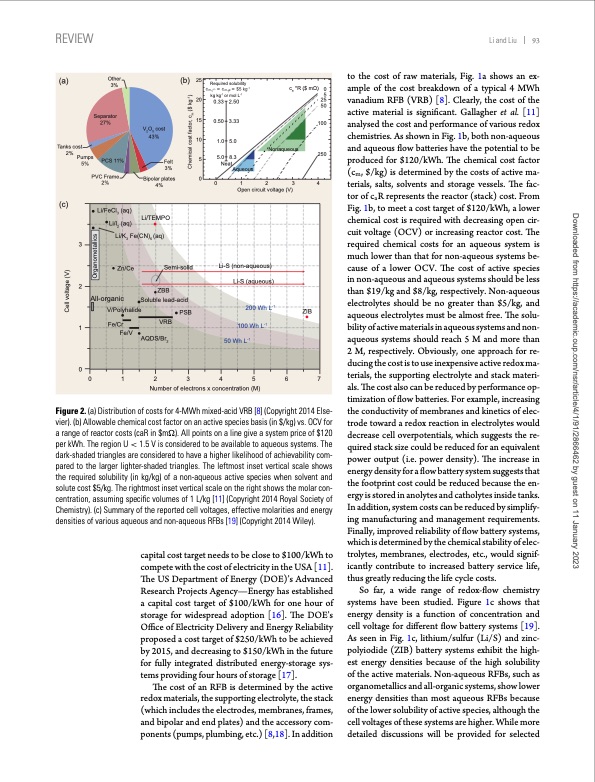
REVIEW Li and Liu 93 (a) Tanks cost 2% Pumps 5% 3 2 1 Other 3% Separator 27% (b) 25 20 15 10 5 (c) Open circuit voltage (V) i-S (n V O cost 25 43% PCS 11% Felt 3% PVC Frame 2% 4% 01234 Bipolar plates 0 Li/FeCl (aq) 3 Li/l (aq) 2 Li/K Fe(CN) (aq) 36 All-organic Zn/Ce V/Polyhalide Fe/Cr Fe/V Li/TEMPO AQDS/Br 2 Semi-solid ZBB Soluble lead-acid VRB PSB L on-aq 50 Wh L-1 100 Wh L-1 ueous) Li-S (aqueous) -1 ZIB 200 Wh L 0 01234567 Number of electrons x concentration (M) Required solubility kg kg-1 or mol L-1 0.33 2.50 0.50 3.33 1.0 5.0 5.0 8.3 Neat Aqueous ca*R($mΩ) 0 5 25 50 Nonaqueous 100 250 Figure 2. (a) Distribution of costs for 4-MWh mixed-acid VRB [8] (Copyright 2014 Else- vier). (b) Allowable chemical cost factor on an active species basis (in $/kg) vs. OCV for a range of reactor costs (caR in $m). All points on a line give a system price of $120 per kWh. The region U < 1.5 V is considered to be available to aqueous systems. The dark-shaded triangles are considered to have a higher likelihood of achievability com- pared to the larger lighter-shaded triangles. The leftmost inset vertical scale shows the required solubility (in kg/kg) of a non-aqueous active species when solvent and solute cost $5/kg. The rightmost inset vertical scale on the right shows the molar con- centration, assuming specific volumes of 1 L/kg [11] (Copyright 2014 Royal Society of Chemistry). (c) Summary of the reported cell voltages, effective molarities and energy densities of various aqueous and non-aqueous RFBs [19] (Copyright 2014 Wiley). capital cost target needs to be close to $100/kWh to compete with the cost of electricity in the USA [11]. The US Department of Energy (DOE)’s Advanced Research Projects Agency—Energy has established a capital cost target of $100/kWh for one hour of storage for widespread adoption [16]. The DOE’s Office of Electricity Delivery and Energy Reliability proposed a cost target of $250/kWh to be achieved by 2015, and decreasing to $150/kWh in the future for fully integrated distributed energy-storage sys- tems providing four hours of storage [17]. The cost of an RFB is determined by the active redox materials, the supporting electrolyte, the stack (which includes the electrodes, membranes, frames, and bipolar and end plates) and the accessory com- ponents (pumps, plumbing, etc.) [8,18]. In addition to the cost of raw materials, Fig. 1a shows an ex- ample of the cost breakdown of a typical 4 MWh vanadium RFB (VRB) [8]. Clearly, the cost of the active material is significant. Gallagher et al. [11] analysed the cost and performance of various redox chemistries. As shown in Fig. 1b, both non-aqueous and aqueous flow batteries have the potential to be produced for $120/kWh. The chemical cost factor (cm, $/kg) is determined by the costs of active ma- terials, salts, solvents and storage vessels. The fac- tor of caR represents the reactor (stack) cost. From Fig. 1b, to meet a cost target of $120/kWh, a lower chemical cost is required with decreasing open cir- cuit voltage (OCV) or increasing reactor cost. The required chemical costs for an aqueous system is much lower than that for non-aqueous systems be- cause of a lower OCV. The cost of active species in non-aqueous and aqueous systems should be less than $19/kg and $8/kg, respectively. Non-aqueous electrolytes should be no greater than $5/kg, and aqueous electrolytes must be almost free. The solu- bility of active materials in aqueous systems and non- aqueous systems should reach 5 M and more than 2 M, respectively. Obviously, one approach for re- ducing the cost is to use inexpensive active redox ma- terials, the supporting electrolyte and stack materi- als. The cost also can be reduced by performance op- timization of flow batteries. For example, increasing the conductivity of membranes and kinetics of elec- trode toward a redox reaction in electrolytes would decrease cell overpotentials, which suggests the re- quired stack size could be reduced for an equivalent power output (i.e. power density). The increase in energy density for a flow battery system suggests that the footprint cost could be reduced because the en- ergy is stored in anolytes and catholytes inside tanks. In addition, system costs can be reduced by simplify- ing manufacturing and management requirements. Finally, improved reliability of flow battery systems, which is determined by the chemical stability of elec- trolytes, membranes, electrodes, etc., would signif- icantly contribute to increased battery service life, thus greatly reducing the life cycle costs. So far, a wide range of redox-flow chemistry systems have been studied. Figure 1c shows that energy density is a function of concentration and cell voltage for different flow battery systems [19]. As seen in Fig. 1c, lithium/sulfur (Li/S) and zinc- polyiodide (ZIB) battery systems exhibit the high- est energy densities because of the high solubility of the active materials. Non-aqueous RFBs, such as organometallics and all-organic systems, show lower energy densities than most aqueous RFBs because of the lower solubility of active species, although the cell voltages of these systems are higher. While more detailed discussions will be provided for selected Cell voltage (V) Organometallics Chemical cost factor, c ($ kg-1) m Downloaded from https://academic.oup.com/nsr/article/4/1/91/2866462 by guest on 11 January 2023PDF Image | Progress in low cost redox flow batteries energy storage
PDF Search Title:
Progress in low cost redox flow batteries energy storageOriginal File Name Searched:
nww098.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |