
PDF Publication Title:
Text from PDF Page: 024
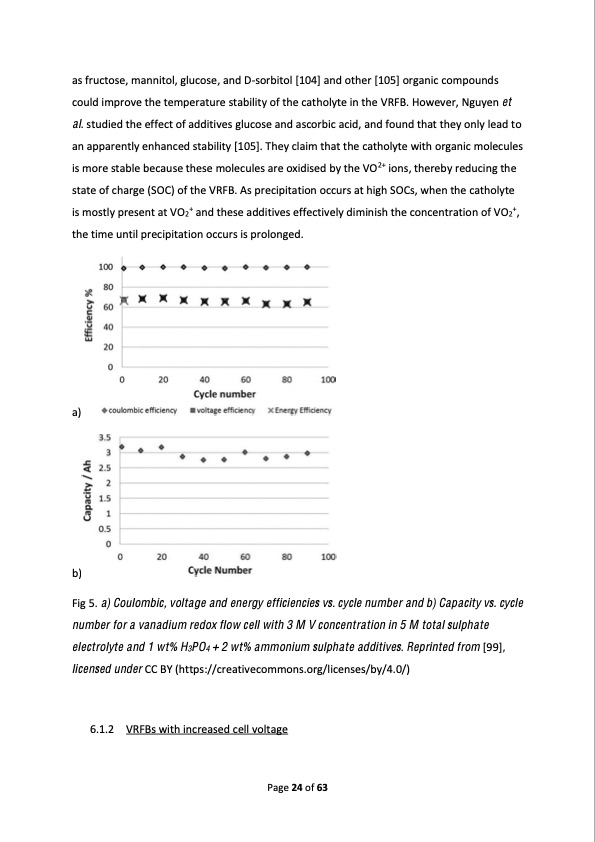
as fructose, mannitol, glucose, and D-sorbitol [104] and other [105] organic compounds could improve the temperature stability of the catholyte in the VRFB. However, Nguyen et al. studied the effect of additives glucose and ascorbic acid, and found that they only lead to an apparently enhanced stability [105]. They claim that the catholyte with organic molecules is more stable because these molecules are oxidised by the VO2+ ions, thereby reducing the state of charge (SOC) of the VRFB. As precipitation occurs at high SOCs, when the catholyte is mostly present at VO2+ and these additives effectively diminish the concentration of VO2+, the time until precipitation occurs is prolonged. a) b) Fig 5. a) Coulombic, voltage and energy efficiencies vs. cycle number and b) Capacity vs. cycle number for a vanadium redox flow cell with 3 M V concentration in 5 M total sulphate electrolyte and 1 wt% H3PO4 + 2 wt% ammonium sulphate additives. Reprinted from [99], licensed under CC BY (https://creativecommons.org/licenses/by/4.0/) 6.1.2 VRFBs with increased cell voltage Page 24 of 63PDF Image | Redox Flow Batteries Concepts Chemistries
PDF Search Title:
Redox Flow Batteries Concepts ChemistriesOriginal File Name Searched:
5870EAF5-2D70-44C8-A0A7-62D3A1462269.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |