
PDF Publication Title:
Text from PDF Page: 088
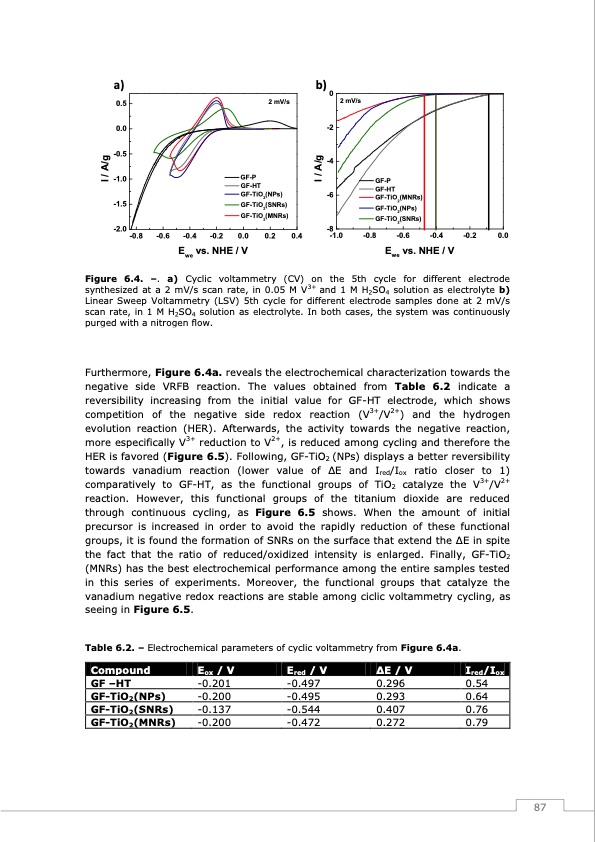
a) b)0 2 mV/s 0.5 0.0 -0.5 -1.0 -1.5 -2.0 2 mV/s -2 -4 I / A/g I / A/g GF-P GF-HT GF-TiO2(NPs) -6 GF-TiO2(SNRs) GF-TiO2(MNRs) GF-P GF-HT GF-TiO2(MNRs) GF-TiO2(NPs) GF-TiO2(SNRs) -8 -0.8 -0.6 -0.4 -0.2 0.0 0.2 0.4 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 Ewe vs. NHE / V Ewe vs. NHE / V Figure 6.4. –. a) Cyclic voltammetry (CV) on the 5th cycle for different electrode synthesized at a 2 mV/s scan rate, in 0.05 M V3+ and 1 M H2SO4 solution as electrolyte b) Linear Sweep Voltammetry (LSV) 5th cycle for different electrode samples done at 2 mV/s scan rate, in 1 M H2SO4 solution as electrolyte. In both cases, the system was continuously purged with a nitrogen flow. Furthermore, Figure 6.4a. reveals the electrochemical characterization towards the negative side VRFB reaction. The values obtained from Table 6.2 indicate a reversibility increasing from the initial value for GF-HT electrode, which shows competition of the negative side redox reaction (V3+/V2+) and the hydrogen evolution reaction (HER). Afterwards, the activity towards the negative reaction, more especifically V3+ reduction to V2+, is reduced among cycling and therefore the HER is favored (Figure 6.5). Following, GF-TiO2 (NPs) displays a better reversibility towards vanadium reaction (lower value of ΔE and Ired/Iox ratio closer to 1) comparatively to GF-HT, as the functional groups of TiO2 catalyze the V3+/V2+ reaction. However, this functional groups of the titanium dioxide are reduced through continuous cycling, as Figure 6.5 shows. When the amount of initial precursor is increased in order to avoid the rapidly reduction of these functional groups, it is found the formation of SNRs on the surface that extend the ΔE in spite the fact that the ratio of reduced/oxidized intensity is enlarged. Finally, GF-TiO2 (MNRs) has the best electrochemical performance among the entire samples tested in this series of experiments. Moreover, the functional groups that catalyze the vanadium negative redox reactions are stable among ciclic voltammetry cycling, as seeing in Figure 6.5. Table 6.2. – Electrochemical parameters of cyclic voltammetry from Figure 6.4a. Compound GF –HT GF-TiO2(NPs) GF-TiO2(SNRs) GF-TiO2(MNRs) Eox / V -0.201 -0.200 -0.137 -0.200 Ered/V ΔE / V Ired/Iox -0.497 0.296 0.54 -0.495 0.293 0.64 -0.544 0.407 0.76 -0.472 0.272 0.79 87PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |