
PDF Publication Title:
Text from PDF Page: 089
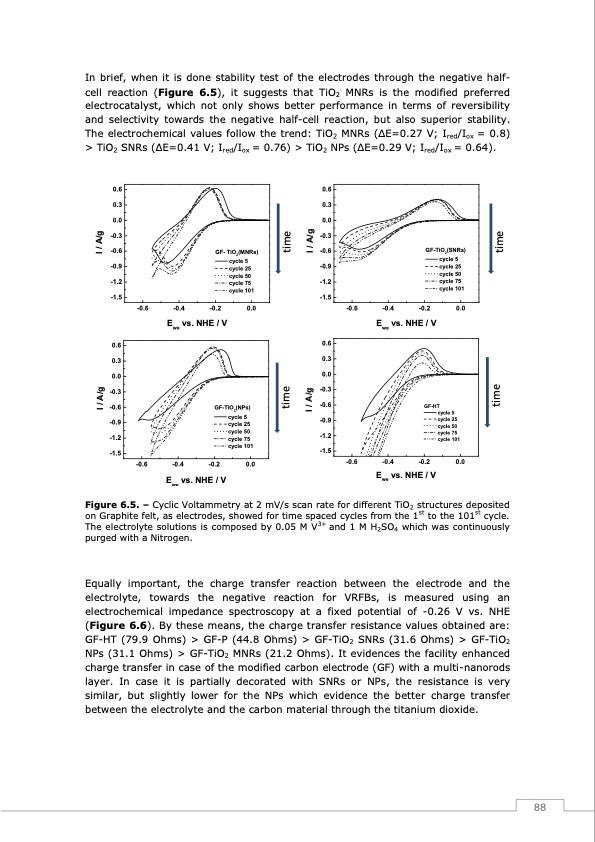
In brief, when it is done stability test of the electrodes through the negative half- cell reaction (Figure 6.5), it suggests that TiO2 MNRs is the modified preferred electrocatalyst, which not only shows better performance in terms of reversibility and selectivity towards the negative half-cell reaction, but also superior stability. The electrochemical values follow the trend: TiO2 MNRs (ΔE=0.27 V; Ired/Iox = 0.8) > TiO2 SNRs (ΔE=0.41 V; Ired/Iox = 0.76) > TiO2 NPs (ΔE=0.29 V; Ired/Iox = 0.64). 0.6 0.3 0.0 -0.3 -0.6 -0.9 -1.2 -1.5 -0.6 GF- TiO (MNRs) 2 0.6 0.3 0.0 -0.3 -0.6 -0.9 -1.2 -1.5 0.6 0.3 0.0 GF-TiO2(SNRs) cycle 5 cycle 25 cycle 50 cycle 75 cycle 101 -0.4 -0.2 0.0 Ewe vs. NHE / V 0.6 0.3 0.0 -0.3 -0.6 -0.9 -1.2 -1.5 GF-TiO (NPs) 2 cycle 5 cycle 25 cycle 50 cycle 75 cycle 101 -0.2 0.0 -0.3 -0.6 GF-HT cycle 101 -1.5 -0.6 -0.4 -0.2 0.0 Ewe vs. NHE / V -0.4 -0.2 cycle 5 cycle 25 cycle 50 cycle 75 cycle 101 0.0 -0.6 Ewe vs. NHE / V I / A/g I / A/g time time I / A/g I / A/g time time -0.6 -0.4 Ewe vs. NHE / V Figure 6.5. – Cyclic Voltammetry at 2 mV/s scan rate for different TiO2 structures deposited on Graphite felt, as electrodes, showed for time spaced cycles from the 1st to the 101st cycle. The electrolyte solutions is composed by 0.05 M V3+ and 1 M H2SO4 which was continuously purged with a Nitrogen. Equally important, the charge transfer reaction between the electrode and the electrolyte, towards the negative reaction for VRFBs, is measured using an electrochemical impedance spectroscopy at a fixed potential of -0.26 V vs. NHE (Figure 6.6). By these means, the charge transfer resistance values obtained are: GF-HT (79.9 Ohms) > GF-P (44.8 Ohms) > GF-TiO2 SNRs (31.6 Ohms) > GF-TiO2 NPs (31.1 Ohms) > GF-TiO2 MNRs (21.2 Ohms). It evidences the facility enhanced charge transfer in case of the modified carbon electrode (GF) with a multi-nanorods layer. In case it is partially decorated with SNRs or NPs, the resistance is very similar, but slightly lower for the NPs which evidence the better charge transfer between the electrolyte and the carbon material through the titanium dioxide. -0.9 -1.2 cycle 75 cycle 5 cycle 25 cycle 50 88PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |