
PDF Publication Title:
Text from PDF Page: 097
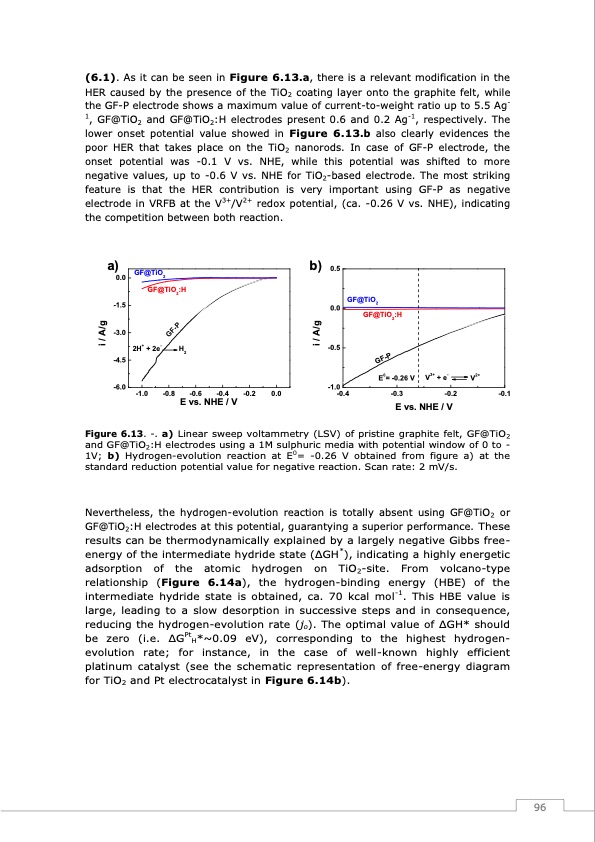
(6.1). As it can be seen in Figure 6.13.a, there is a relevant modification in the HER caused by the presence of the TiO2 coating layer onto the graphite felt, while the GF-P electrode shows a maximum value of current-to-weight ratio up to 5.5 Ag- 1, GF@TiO2 and GF@TiO2:H electrodes present 0.6 and 0.2 Ag-1, respectively. The lower onset potential value showed in Figure 6.13.b also clearly evidences the poor HER that takes place on the TiO2 nanorods. In case of GF-P electrode, the onset potential was -0.1 V vs. NHE, while this potential was shifted to more negative values, up to -0.6 V vs. NHE for TiO2-based electrode. The most striking feature is that the HER contribution is very important using GF-P as negative electrode in VRFB at the V3+/V2+ redox potential, (ca. -0.26 V vs. NHE), indicating the competition between both reaction. a) 0.0 -1.5 -3.0 -4.5 -6.0 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 b) 0.5 0.0 -0.5 -1.0 -0.4 -0.3 -0.2 -0.1 E vs. NHE / V GF@TiO2 GF@TiO2:H 2H+ + 2e- H2 GF@TiO2 GF@TiO2:H E0= -0.26 V V3+ + e- V2+ E vs. NHE / V Figure 6.13. -. a) Linear sweep voltammetry (LSV) of pristine graphite felt, GF@TiO2 and GF@TiO2:H electrodes using a 1M sulphuric media with potential window of 0 to - 1V; b) Hydrogen-evolution reaction at E0= -0.26 V obtained from figure a) at the standard reduction potential value for negative reaction. Scan rate: 2 mV/s. Nevertheless, the hydrogen-evolution reaction is totally absent using GF@TiO2 or GF@TiO2:H electrodes at this potential, guarantying a superior performance. These results can be thermodynamically explained by a largely negative Gibbs free- energy of the intermediate hydride state (ΔGH*), indicating a highly energetic adsorption of the atomic hydrogen on TiO2-site. From volcano-type relationship (Figure 6.14a), the hydrogen-binding energy (HBE) of the intermediate hydride state is obtained, ca. 70 kcal mol-1. This HBE value is large, leading to a slow desorption in successive steps and in consequence, reducing the hydrogen-evolution rate (jo). The optimal value of ΔGH* should be zero (i.e. ΔGPtH*~0.09 eV), corresponding to the highest hydrogen- evolution rate; for instance, in the case of well-known highly efficient platinum catalyst (see the schematic representation of free-energy diagram for TiO2 and Pt electrocatalyst in Figure 6.14b). 96 i / A/g i / A/g GF-P GF-PPDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |