
PDF Publication Title:
Text from PDF Page: 098
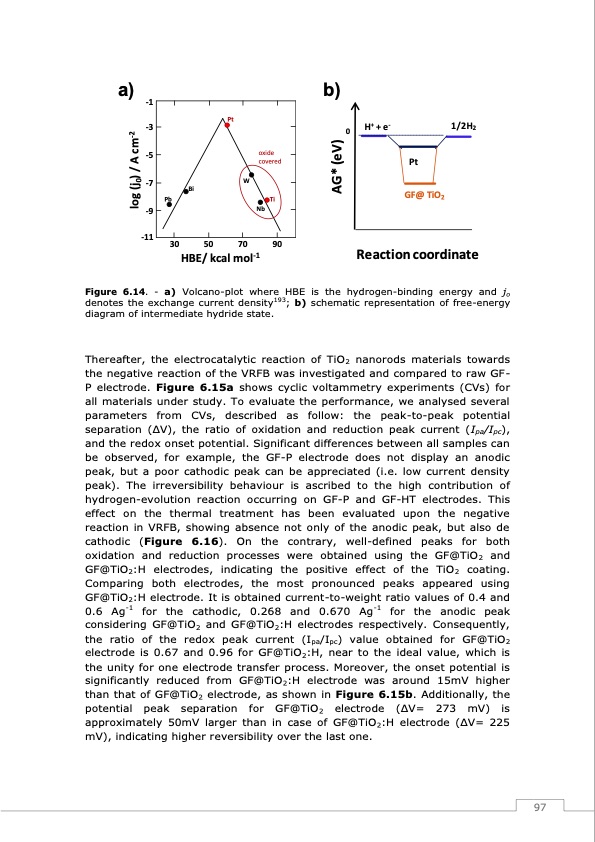
a) -1 b) -3 0 H+ + e- 1/2H2 -5 -7 -9 -11 Pt GF@ TiO2 Pb Ti Bi 30 50 70 90 HBE/ kcal mol-1 Reaction coordinate Pt W Nb oxide covered Figure 6.14. - a) Volcano-plot where HBE is the hydrogen-binding energy and jo denotes the exchange current density193; b) schematic representation of free-energy diagram of intermediate hydride state. Thereafter, the electrocatalytic reaction of TiO2 nanorods materials towards the negative reaction of the VRFB was investigated and compared to raw GF- P electrode. Figure 6.15a shows cyclic voltammetry experiments (CVs) for all materials under study. To evaluate the performance, we analysed several parameters from CVs, described as follow: the peak-to-peak potential separation (ΔV), the ratio of oxidation and reduction peak current (Ipa/Ipc), and the redox onset potential. Significant differences between all samples can be observed, for example, the GF-P electrode does not display an anodic peak, but a poor cathodic peak can be appreciated (i.e. low current density peak). The irreversibility behaviour is ascribed to the high contribution of hydrogen-evolution reaction occurring on GF-P and GF-HT electrodes. This effect on the thermal treatment has been evaluated upon the negative reaction in VRFB, showing absence not only of the anodic peak, but also de cathodic (Figure 6.16). On the contrary, well-defined peaks for both oxidation and reduction processes were obtained using the GF@TiO2 and GF@TiO2:H electrodes, indicating the positive effect of the TiO2 coating. Comparing both electrodes, the most pronounced peaks appeared using GF@TiO2:H electrode. It is obtained current-to-weight ratio values of 0.4 and 0.6 Ag-1 for the cathodic, 0.268 and 0.670 Ag-1 for the anodic peak considering GF@TiO2 and GF@TiO2:H electrodes respectively. Consequently, the ratio of the redox peak current (Ipa/Ipc) value obtained for GF@TiO2 electrode is 0.67 and 0.96 for GF@TiO2:H, near to the ideal value, which is the unity for one electrode transfer process. Moreover, the onset potential is significantly reduced from GF@TiO2:H electrode was around 15mV higher than that of GF@TiO2 electrode, as shown in Figure 6.15b. Additionally, the potential peak separation for GF@TiO2 electrode (ΔV= 273 mV) is approximately 50mV larger than in case of GF@TiO2:H electrode (ΔV= 225 mV), indicating higher reversibility over the last one. 97 log (j0) / A cm-2 AG* (eV)PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |