
PDF Publication Title:
Text from PDF Page: 107
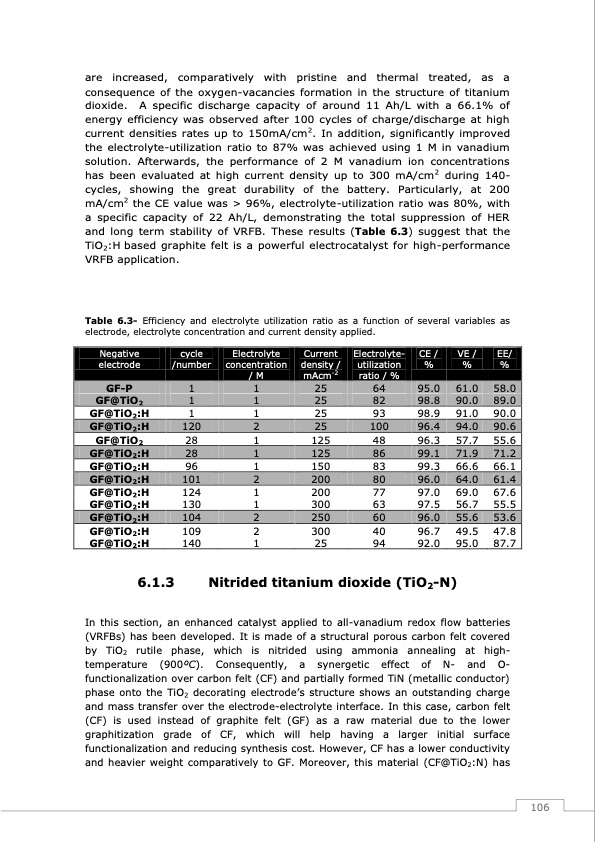
are increased, comparatively with pristine and thermal treated, as a consequence of the oxygen-vacancies formation in the structure of titanium dioxide. A specific discharge capacity of around 11 Ah/L with a 66.1% of energy efficiency was observed after 100 cycles of charge/discharge at high current densities rates up to 150mA/cm2. In addition, significantly improved the electrolyte-utilization ratio to 87% was achieved using 1 M in vanadium solution. Afterwards, the performance of 2 M vanadium ion concentrations has been evaluated at high current density up to 300 mA/cm2 during 140- cycles, showing the great durability of the battery. Particularly, at 200 mA/cm2 the CE value was > 96%, electrolyte-utilization ratio was 80%, with a specific capacity of 22 Ah/L, demonstrating the total suppression of HER and long term stability of VRFB. These results (Table 6.3) suggest that the TiO2:H based graphite felt is a powerful electrocatalyst for high-performance VRFB application. Table 6.3- Efficiency and electrolyte utilization ratio as a function of several variables as electrode, electrolyte concentration and current density applied. Negative electrode cycle /number Electrolyte concentration /M Current density / mAcm-2 Electrolyte- utilization ratio / % CE / % VE / % GF-P GF@TiO2 GF@TiO2:H GF@TiO2:H GF@TiO2:H GF@TiO2:H GF@TiO2:H 6.1.3 1 1 124 1 130 1 104 2 109 2 140 1 EE/ % 95.0 58.0 25 82 98.8 90.0 89.0 90.0 90.6 55.6 71.2 66.1 96.0 64.0 61.4 200 77 97.0 69.0 67.6 300 63 97.5 56.7 55.5 250 60 96.0 55.6 53.6 300 40 96.7 49.5 47.8 25 94 92.0 95.0 87.7 1 1 25 64 61.0 GF@TiO2:H 1 1 25 93 98.9 91.0 GF@TiO2:H 120 2 25 100 96.4 94.0 GF@TiO2 28 1 125 48 96.3 57.7 GF@TiO2:H 28 1 125 86 99.1 71.9 GF@TiO2:H 96 1 150 83 99.3 66.6 GF@TiO2:H 101 2 200 80 Nitrided titanium dioxide (TiO2-N) In this section, an enhanced catalyst applied to all-vanadium redox flow batteries (VRFBs) has been developed. It is made of a structural porous carbon felt covered by TiO2 rutile phase, which is nitrided using ammonia annealing at high- temperature (900oC). Consequently, a synergetic effect of N- and O- functionalization over carbon felt (CF) and partially formed TiN (metallic conductor) phase onto the TiO2 decorating electrode’s structure shows an outstanding charge and mass transfer over the electrode-electrolyte interface. In this case, carbon felt (CF) is used instead of graphite felt (GF) as a raw material due to the lower graphitization grade of CF, which will help having a larger initial surface functionalization and reducing synthesis cost. However, CF has a lower conductivity and heavier weight comparatively to GF. Moreover, this material (CF@TiO2:N) has 106PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |