
PDF Publication Title:
Text from PDF Page: 218
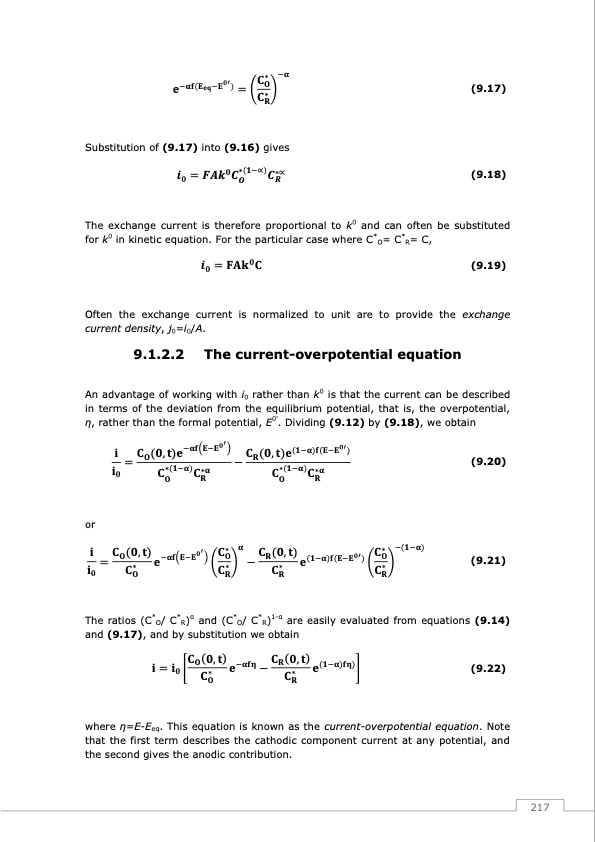
Substitution of (9.17) into (9.16) gives (9.17) (9.18) The exchange current is therefore proportional to k0 and can often be substituted for k0 in kinetic equation. For the particular case where C*O= C*R= C, (9.19) Often the exchange current is normalized to unit are to provide the exchange current density, j0=i0/A. 9.1.2.2 The current-overpotential equation An advantage of working with i0 rather than k0 is that the current can be described in terms of the deviation from the equilibrium potential, that is, the overpotential, η, rather than the formal potential, E0’. Dividing (9.12) by (9.18), we obtain or (9.20) (9.21) The ratios (C*O/ C*R)α and (C*O/ C*R)1-α are easily evaluated from equations (9.14) and (9.17), and by substitution we obtain (9.22) where η=E-Eeq. This equation is known as the current-overpotential equation. Note that the first term describes the cathodic component current at any potential, and the second gives the anodic contribution. 217PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |