
PDF Publication Title:
Text from PDF Page: 004
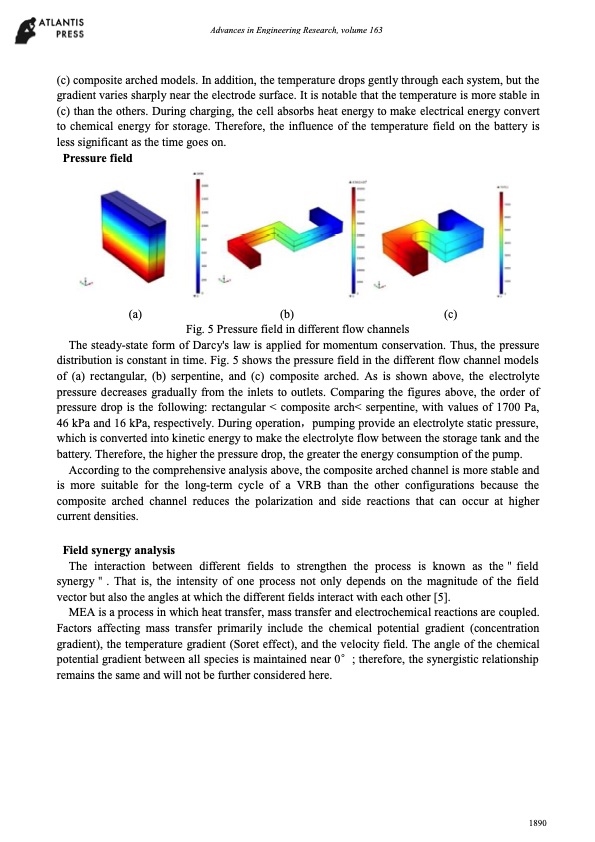
Advances in Engineering Research, volume 163 (c) composite arched models. In addition, the temperature drops gently through each system, but the gradient varies sharply near the electrode surface. It is notable that the temperature is more stable in (c) than the others. During charging, the cell absorbs heat energy to make electrical energy convert to chemical energy for storage. Therefore, the influence of the temperature field on the battery is less significant as the time goes on. Pressure field (a) (b) (c) Fig. 5 Pressure field in different flow channels The steady-state form of Darcy's law is applied for momentum conservation. Thus, the pressure distribution is constant in time. Fig. 5 shows the pressure field in the different flow channel models of (a) rectangular, (b) serpentine, and (c) composite arched. As is shown above, the electrolyte pressure decreases gradually from the inlets to outlets. Comparing the figures above, the order of pressure drop is the following: rectangular < composite arch< serpentine, with values of 1700 Pa, 46 kPa and 16 kPa, respectively. During operation,pumping provide an electrolyte static pressure, which is converted into kinetic energy to make the electrolyte flow between the storage tank and the battery. Therefore, the higher the pressure drop, the greater the energy consumption of the pump. According to the comprehensive analysis above, the composite arched channel is more stable and is more suitable for the long-term cycle of a VRB than the other configurations because the composite arched channel reduces the polarization and side reactions that can occur at higher current densities. Field synergy analysis The interaction between different fields to strengthen the process is known as the"field synergy". That is, the intensity of one process not only depends on the magnitude of the field vector but also the angles at which the different fields interact with each other [5]. MEA is a process in which heat transfer, mass transfer and electrochemical reactions are coupled. Factors affecting mass transfer primarily include the chemical potential gradient (concentration gradient), the temperature gradient (Soret effect), and the velocity field. The angle of the chemical potential gradient between all species is maintained near 0°; therefore, the synergistic relationship remains the same and will not be further considered here. 1890PDF Image | Simulation all-vanadium redox flow battery arched channel
PDF Search Title:
Simulation all-vanadium redox flow battery arched channelOriginal File Name Searched:
25894955.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |