
PDF Publication Title:
Text from PDF Page: 003
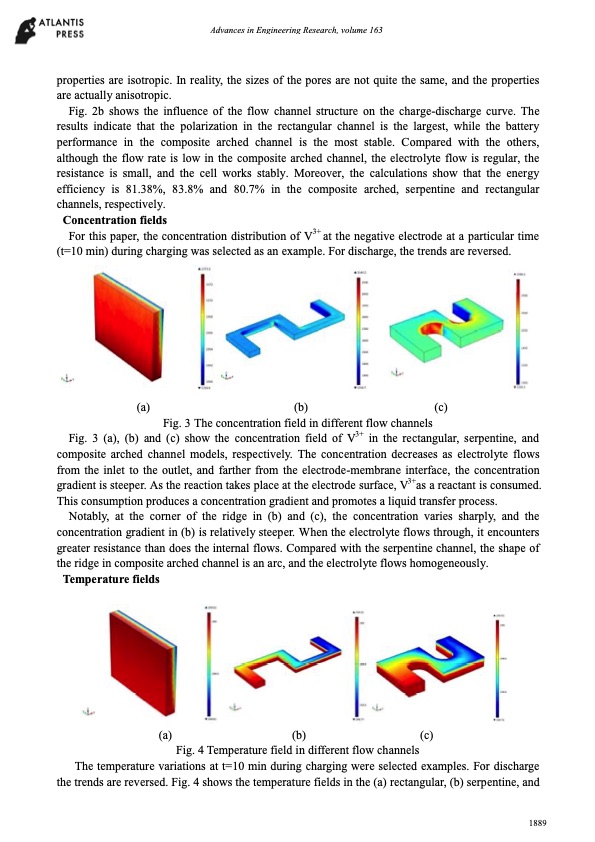
Advances in Engineering Research, volume 163 properties are isotropic. In reality, the sizes of the pores are not quite the same, and the properties are actually anisotropic. Fig. 2b shows the influence of the flow channel structure on the charge-discharge curve. The results indicate that the polarization in the rectangular channel is the largest, while the battery performance in the composite arched channel is the most stable. Compared with the others, although the flow rate is low in the composite arched channel, the electrolyte flow is regular, the resistance is small, and the cell works stably. Moreover, the calculations show that the energy efficiency is 81.38%, 83.8% and 80.7% in the composite arched, serpentine and rectangular channels, respectively. Concentration fields For this paper, the concentration distribution of V3+ at the negative electrode at a particular time (t=10 min) during charging was selected as an example. For discharge, the trends are reversed. (a) (b) (c) Fig. 3 The concentration field in different flow channels Fig. 3 (a), (b) and (c) show the concentration field of V3+ in the rectangular, serpentine, and composite arched channel models, respectively. The concentration decreases as electrolyte flows from the inlet to the outlet, and farther from the electrode-membrane interface, the concentration gradient is steeper. As the reaction takes place at the electrode surface, V3+as a reactant is consumed. This consumption produces a concentration gradient and promotes a liquid transfer process. Notably, at the corner of the ridge in (b) and (c), the concentration varies sharply, and the concentration gradient in (b) is relatively steeper. When the electrolyte flows through, it encounters greater resistance than does the internal flows. Compared with the serpentine channel, the shape of the ridge in composite arched channel is an arc, and the electrolyte flows homogeneously. Temperature fields (a) (b) (c) Fig. 4 Temperature field in different flow channels The temperature variations at t=10 min during charging were selected examples. For discharge the trends are reversed. Fig. 4 shows the temperature fields in the (a) rectangular, (b) serpentine, and 1889PDF Image | Simulation all-vanadium redox flow battery arched channel
PDF Search Title:
Simulation all-vanadium redox flow battery arched channelOriginal File Name Searched:
25894955.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |