
PDF Publication Title:
Text from PDF Page: 010
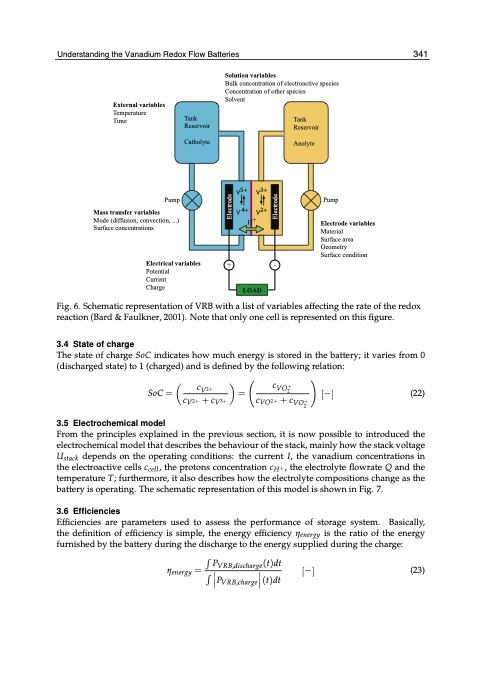
UnderstandininggththeeVVaannaaddiuimumRRedeodxoFxloFwlowBaBttaetrtiesries 3491 External variables Solution variables Bulk concentration of electroactive species Concentration of other species Solvent Tank Reservoir Anolyte Temperature Time Mass transfer variables Tank Reservoir Catholyte Mode (diffusion, convection, ...) Surface concentrations Electrical variables + Potential Current Charge - Pump Pump Electrode variables Material Surface area Geometry Surface condition ��� ��� ��� ��� H+ LOAD Fig. 6. Schematic representation of VRB with a list of variables affecting the rate of the redox reaction (Bard & Faulkner, 2001). Note that only one cell is represented on this figure. 3.4 State of charge The state of charge SoC indicates how much energy is stored in the battery; it varies from 0 (discharged state) to 1 (charged) and is defined by the following relation: SoC = 3.5 Electrochemical model c V 2 + cV2+ +cV3+ c V O 2+ = [−] (22) From the principles explained in the previous section, it is now possible to introduced the electrochemical model that describes the behaviour of the stack, mainly how the stack voltage Ustack depends on the operating conditions: the current I, the vanadium concentrations in the electroactive cells ccell, the protons concentration cH+, the electrolyte flowrate Q and the temperature T; furthermore, it also describes how the electrolyte compositions change as the battery is operating. The schematic representation of this model is shown in Fig. 7. 3.6 Efficiencies Efficiencies are parameters used to assess the performance of storage system. Basically, the definition of efficiency is simple, the energy efficiency ηenergy is the ratio of the energy furnished by the battery during the discharge to the energy supplied during the charge: PVRB,discharge(t)dt ηenergy = [−] (23) PVRB,charge(t)dt cVO2+ +cVO2+ Electrode ElectrodePDF Image | Understanding the Vanadium Redox Flow Batteries
PDF Search Title:
Understanding the Vanadium Redox Flow BatteriesOriginal File Name Searched:
12523.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |