
PDF Publication Title:
Text from PDF Page: 011
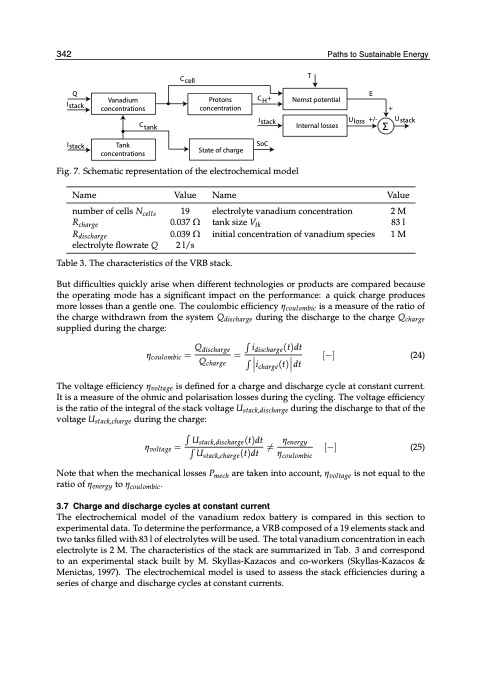
31042 I stack Paths tSouSsutastianianbalbeleEEnneergrgyy Q CH+ E I stack Vanadium concentrations Name number of cells Ncells Rcharge Rdischarge electrolyte flowrate Q Value Name Value 2 M 83 l 1 M Ctank Istack SoC Uloss +/- Σ Ustack C cell T ηcoulombic= Q charge Protons concentration Qdischarge idischarge (t)dt icharge (t) dt ηvoltage = U (t)dt Ustack,discharge (t)dt stack,charge ηenergy coulombic [−] (25) Nernst potential + Internal losses Tank concentrations Fig. 7. Schematic representation of the electrochemical model State of charge 19 0.037 Ω 0.039 Ω 2 l/s electrolyte vanadium concentration tank size Vtk initial concentration of vanadium species Table 3. The characteristics of the VRB stack. But difficulties quickly arise when different technologies or products are compared because the operating mode has a significant impact on the performance: a quick charge produces more losses than a gentle one. The coulombic efficiency ηcoulombic is a measure of the ratio of the charge withdrawn from the system Qdischarge during the discharge to the charge Qcharge supplied during the charge: = [−] (24) The voltage efficiency ηvoltage is defined for a charge and discharge cycle at constant current. It is a measure of the ohmic and polarisation losses during the cycling. The voltage efficiency is the ratio of the integral of the stack voltage Ustack,discharge during the discharge to that of the voltage Ustack,charge during the charge: ̸= η Note that when the mechanical losses Pmech are taken into account, ηvoltage is not equal to the ratio of ηenergy to ηcoulombic. 3.7 Charge and discharge cycles at constant current The electrochemical model of the vanadium redox battery is compared in this section to experimental data. To determine the performance, a VRB composed of a 19 elements stack and two tanks filled with 83 l of electrolytes will be used. The total vanadium concentration in each electrolyte is 2 M. The characteristics of the stack are summarized in Tab. 3 and correspond to an experimental stack built by M. Skyllas-Kazacos and co-workers (Skyllas-Kazacos & Menictas, 1997). The electrochemical model is used to assess the stack efficiencies during a series of charge and discharge cycles at constant currents.PDF Image | Understanding the Vanadium Redox Flow Batteries
PDF Search Title:
Understanding the Vanadium Redox Flow BatteriesOriginal File Name Searched:
12523.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |