
PDF Publication Title:
Text from PDF Page: 010
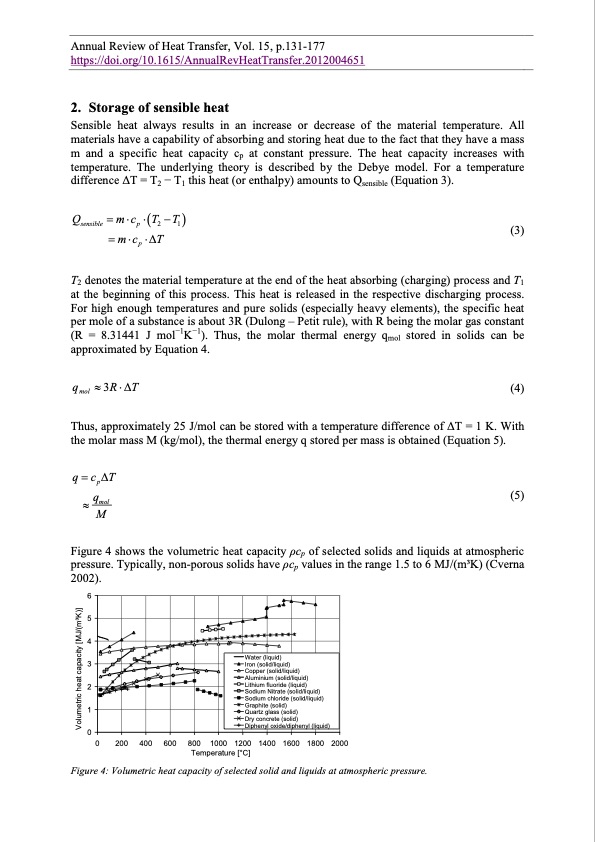
Annual Review of Heat Transfer, Vol. 15, p.131-177 https://doi.org/10.1615/AnnualRevHeatTransfer.2012004651 2. Storage of sensible heat Sensible heat always results in an increase or decrease of the material temperature. All materials have a capability of absorbing and storing heat due to the fact that they have a mass m and a specific heat capacity cp at constant pressure. The heat capacity increases with temperature. The underlying theory is described by the Debye model. For a temperature difference ΔT = T2 − T1 this heat (or enthalpy) amounts to Qsensible (Equation 3). Q mc T T sensible p 2 1 mcp T (3) T2 denotes the material temperature at the end of the heat absorbing (charging) process and T1 at the beginning of this process. This heat is released in the respective discharging process. For high enough temperatures and pure solids (especially heavy elements), the specific heat per mole of a substance is about 3R (Dulong – Petit rule), with R being the molar gas constant (R = 8.31441 J mol−1K−1). Thus, the molar thermal energy qmol stored in solids can be approximated by Equation 4. qmol 3RT (4) Thus, approximately 25 J/mol can be stored with a temperature difference of ΔT = 1 K. With the molar mass M (kg/mol), the thermal energy q stored per mass is obtained (Equation 5). q cpT qmol M (5) Figure 4 shows the volumetric heat capacity ρcp of selected solids and liquids at atmospheric pressure. Typically, non-porous solids have ρcp values in the range 1.5 to 6 MJ/(m3K) (Cverna 2002). 6 5 4 3 2 1 0 Water (liquid) Iron (solid/liquid) Copper (solid/liquid) Aluminium (solid/liquid) Lithium fluoride (liquid) Sodium Nitrate (solid/liquid) Sodium chloride (solid/liquid) Graphite (solid) Quartz glass (solid) Dry concrete (solid) Diphenyl oxide/diphenyl (liquid) 0 200 400 600 800 1000 1200 1400 1600 1800 2000 Temperature [°C] Figure 4: Volumetric heat capacity of selected solid and liquids at atmospheric pressure. Volumetric heat capacity [MJ/(m3K)]PDF Image | Annual Review of Heat Transfer
PDF Search Title:
Annual Review of Heat TransferOriginal File Name Searched:
2012_Thermal_Energy_Storage_Materials_and_Systems.pdfDIY PDF Search: Google It | Yahoo | Bing
Turbine and System Plans CAD CAM: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. More Info
Waste Heat Power Technology: Organic Rankine Cycle uses waste heat to make electricity, shaft horsepower and cooling. More Info
All Turbine and System Products: Infinity Turbine ORD systems, turbine generator sets, build plans and more to use your waste heat from 30C to 100C. More Info
CO2 Phase Change Demonstrator: CO2 goes supercritical at 30 C. This is a experimental platform which you can use to demonstrate phase change with low heat. Includes integration area for small CO2 turbine, static generator, and more. This can also be used for a GTL Gas to Liquids experimental platform. More Info
Introducing the Infinity Turbine Products Infinity Turbine develops and builds systems for making power from waste heat. It also is working on innovative strategies for storing, making, and deploying energy. More Info
Need Strategy? Use our Consulting and analyst services Infinity Turbine LLC is pleased to announce its consulting and analyst services. We have worked in the renewable energy industry as a researcher, developing sales and markets, along with may inventions and innovations. More Info
Made in USA with Global Energy Millennial Web Engine These pages were made with the Global Energy Web PDF Engine using Filemaker (Claris) software.
Sand Battery Sand and Paraffin for TES Thermo Energy Storage More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |