
PDF Publication Title:
Text from PDF Page: 220
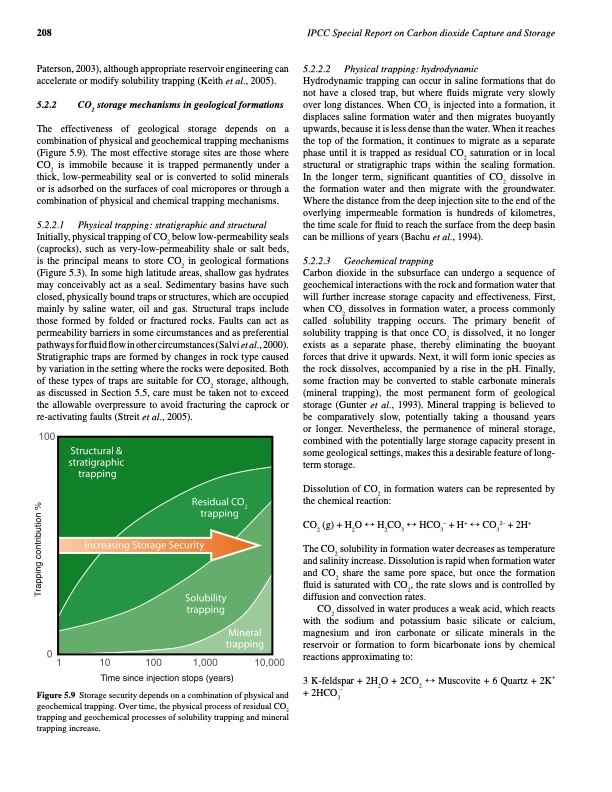
208 IPCC Special Report on Carbon dioxide Capture and Storage Paterson, 2003), although appropriate reservoir engineering can accelerate or modify solubility trapping (Keith et al., 2005). 5.2.2 CO2 storage mechanisms in geological formations The effectiveness of geological storage depends on a combination of physical and geochemical trapping mechanisms (Figure 5.9). The most effective storage sites are those where CO2 is immobile because it is trapped permanently under a thick, low-permeability seal or is converted to solid minerals or is adsorbed on the surfaces of coal micropores or through a combination of physical and chemical trapping mechanisms. Initially, physical trapping of CO2 below low-permeability seals (caprocks), such as very-low-permeability shale or salt beds, is the principal means to store CO2 in geological formations (Figure 5.3). In some high latitude areas, shallow gas hydrates may conceivably act as a seal. Sedimentary basins have such closed, physically bound traps or structures, which are occupied mainly by saline water, oil and gas. Structural traps include those formed by folded or fractured rocks. Faults can act as permeability barriers in some circumstances and as preferential pathways for fluid flow in other circumstances (Salvi et al., 2000). Stratigraphic traps are formed by changes in rock type caused by variation in the setting where the rocks were deposited. Both of these types of traps are suitable for CO2 storage, although, as discussed in Section 5.5, care must be taken not to exceed the allowable overpressure to avoid fracturing the caprock or re-activating faults (Streit et al., 2005). 5.2.2.2 Physical trapping: hydrodynamic Hydrodynamic trapping can occur in saline formations that do not have a closed trap, but where fluids migrate very slowly over long distances. When CO2 is injected into a formation, it displaces saline formation water and then migrates buoyantly upwards, because it is less dense than the water. When it reaches the top of the formation, it continues to migrate as a separate phase until it is trapped as residual CO2 saturation or in local structural or stratigraphic traps within the sealing formation. In the longer term, significant quantities of CO2 dissolve in the formation water and then migrate with the groundwater. Where the distance from the deep injection site to the end of the overlying impermeable formation is hundreds of kilometres, the time scale for fluid to reach the surface from the deep basin can be millions of years (Bachu et al., 1994). 5.2.2.3 Geochemical trapping Carbon dioxide in the subsurface can undergo a sequence of geochemical interactions with the rock and formation water that will further increase storage capacity and effectiveness. First, when CO2 dissolves in formation water, a process commonly called solubility trapping occurs. The primary benefit of solubility trapping is that once CO2 is dissolved, it no longer exists as a separate phase, thereby eliminating the buoyant forces that drive it upwards. Next, it will form ionic species as the rock dissolves, accompanied by a rise in the pH. Finally, some fraction may be converted to stable carbonate minerals (mineral trapping), the most permanent form of geological storage (Gunter et al., 1993). Mineral trapping is believed to be comparatively slow, potentially taking a thousand years or longer. Nevertheless, the permanence of mineral storage, combined with the potentially large storage capacity present in some geological settings, makes this a desirable feature of long- term storage. Dissolution of CO2 in formation waters can be represented by the chemical reaction: CO2 (g) + H2O ↔ H2CO3 ↔ HCO3– + H+ ↔ CO32– + 2H+ 5.2.2.1 Physical trapping: stratigraphic and structural + 2HCO3– geochemical trapping. Over time, the physical process of residual CO2 trapping and geochemical processes of solubility trapping and mineral Figure 5.9 Storage security depends on a combination of physical and trapping increase. The CO2 solubility in formation water decreases as temperature and salinity increase. Dissolution is rapid when formation water and CO2 share the same pore space, but once the formation fluid is saturated with CO2, the rate slows and is controlled by diffusion and convection rates. CO2 dissolved in water produces a weak acid, which reacts with the sodium and potassium basic silicate or calcium, magnesium and iron carbonate or silicate minerals in the reservoir or formation to form bicarbonate ions by chemical reactions approximating to: 3 K-feldspar + 2H2O + 2CO2 ↔ Muscovite + 6 Quartz + 2K+PDF Image | CARBON DIOXIDE CAPTURE AND STORAGE
PDF Search Title:
CARBON DIOXIDE CAPTURE AND STORAGEOriginal File Name Searched:
srccs_wholereport.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |