
PDF Publication Title:
Text from PDF Page: 020
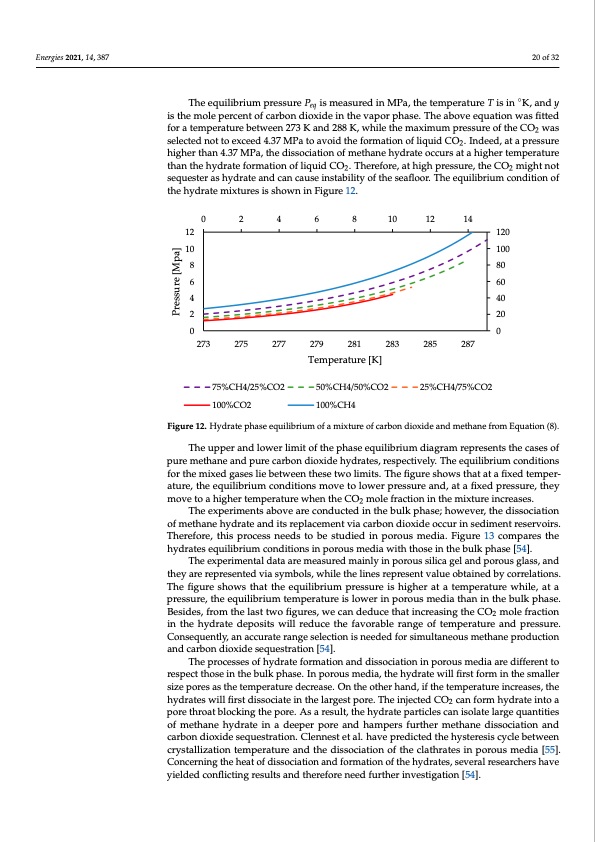
Energies 2021, 14, 387 20 of 32 Energies 2021, 14, x FOR PEER REVIEW The equilibrium pressure Peq is measured in MPa, the temperature T is in ◦21 of 33 K, and y is the mole percent of carbon dioxide in the vapor phase. The above equation was fitted for a temperature between 273 K and 288 K, while the maximum pressure of the CO2 was selected not to exceed 4.37 MPa to avoid the formation of liquid CO2. Indeed, at a pressure selected not to exceed 4.37 MPa to avoid the formation of liquid CO2. Indeed, at a pressure higher than 4.37 MPa, the dissociation of methane hydrate occurs at a higher temperature higher than 4.37 MPa, the dissociation of methane hydrate occurs at a higher temperature than the hydrate formation of liquid CO2. Therefore, at high pressure, the CO2 might not than the hydrate formation of liquid CO2. Therefore, at high pressure, the CO2 might not sequester as hydrate and can cause instability of the seafloor. The equilibrium condition sequester as hydrate and can cause instability of the seafloor. The equilibrium condition of of the hydrate mixtures is shown in Figure 12.. 12 10 8 6 4 2 120 100 80 60 40 20 0 2 4 6 8 10 12 14 00 273 275 277 75%CH4/25%CO2 100%CO2 279 281 283 285 287 Temperature [K] 50%CH4/50%CO2 25%CH4/75%CO2 100%CH4 Figure 12. Hydrate phase equilibrium of a mixture of carbon dioxide and methane from Equation (8). Figure 12. Hydrate phase equilibrium of a mixture of carbon dioxide and methane from Equation (8). The upper and lower limit of the phase equilibrium diagram represents the cases of pure methane and pure carbon dioxide hydrates, respectively. The equilibrium conditions The upper and lower limit of the phase equilibrium diagram represents the cases of for the mixed gases lie between these two limits. The figure shows that at a fixed temper- pure methane and pure carbon dioxide hydrates, respectively. The equilibrium conditions ature, the equilibrium conditions move to lower pressure and, at a fixed pressure, they for the mixed gases lie between these two limits. The figure shows that at a fixed temper- move to a higher temperature when the CO2 mole fraction in the mixture increases. ature, the equilibrium conditions move to lower pressure and, at a fixed pressure, they The experiments above are conducted in the bulk phase; however, the dissociation move to a higher temperature when the CO2 mole fraction in the mixture increases. of methane hydrate and its replacement via carbon dioxide occur in sediment reservoirs. The experiments above are conducted in the bulk phase; however, the dissociation of Therefore, this process needs to be studied in porous media. Figure 13 compares the methane hydrate and its replacement via carbon dioxide occur in sediment reservoirs. hydrates equilibrium conditions in porous media with those in the bulk phase [54]. Therefore, this process needs to be studied in porous media. Figure 13 compares the hy- The experimental data are measured mainly in porous silica gel and porous glass, and drates equilibrium conditions in porous media with those in the bulk phase [54]. they are represented via symbols, while the lines represent value obtained by correlations. The figure shows that the equilibrium pressure is higher at a temperature while, at a pressure, the equilibrium temperature is lower in porous media than in the bulk phase. Besides, from the last two figures, we can deduce that increasing the CO2 mole fraction in the hydrate deposits will reduce the favorable range of temperature and pressure. Consequently, an accurate range selection is needed for simultaneous methane production and carbon dioxide sequestration [54]. The processes of hydrate formation and dissociation in porous media are different to respect those in the bulk phase. In porous media, the hydrate will first form in the smaller size pores as the temperature decrease. On the other hand, if the temperature increases, the hydrates will first dissociate in the largest pore. The injected CO2 can form hydrate into a pore throat blocking the pore. As a result, the hydrate particles can isolate large quantities of methane hydrate in a deeper pore and hampers further methane dissociation and carbon dioxide sequestration. Clennest et al. have predicted the hysteresis cycle between crystallization temperature and the dissociation of the clathrates in porous media [55]. Concerning the heat of dissociation and formation of the hydrates, several researchers have yielded conflicting results and therefore need further investigation [54]. Pressure [Mpa]PDF Image | Energies 14
PDF Search Title:
Energies 14Original File Name Searched:
energies-14-00387.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |