
PDF Publication Title:
Text from PDF Page: 006
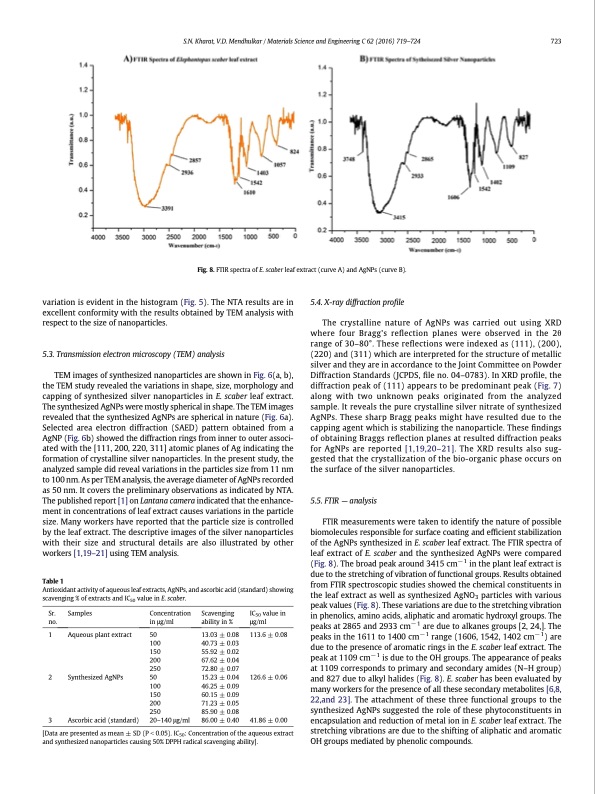
variation is evident in the histogram (Fig. 5). The NTA results are in excellent conformity with the results obtained by TEM analysis with respect to the size of nanoparticles. 5.3. Transmission electron microscopy (TEM) analysis TEM images of synthesized nanoparticles are shown in Fig. 6(a, b), the TEM study revealed the variations in shape, size, morphology and capping of synthesized silver nanoparticles in E. scaber leaf extract. The synthesized AgNPs were mostly spherical in shape. The TEM images revealed that the synthesized AgNPs are spherical in nature (Fig. 6a). Selected area electron diffraction (SAED) pattern obtained from a AgNP (Fig. 6b) showed the diffraction rings from inner to outer associ- ated with the [111, 200, 220, 311] atomic planes of Ag indicating the formation of crystalline silver nanoparticles. In the present study, the analyzed sample did reveal variations in the particles size from 11 nm to 100 nm. As per TEM analysis, the average diameter of AgNPs recorded as 50 nm. It covers the preliminary observations as indicated by NTA. The published report [1] on Lantana camera indicated that the enhance- ment in concentrations of leaf extract causes variations in the particle size. Many workers have reported that the particle size is controlled by the leaf extract. The descriptive images of the silver nanoparticles with their size and structural details are also illustrated by other workers [1,19–21] using TEM analysis. Table 1 Antioxidant activity of aqueous leaf extracts, AgNPs, and ascorbic acid (standard) showing scavenging % of extracts and IC50 value in E. scaber. 5.4. X-ray diffraction profile The crystalline nature of AgNPs was carried out using XRD where four Bragg's reflection planes were observed in the 2θ range of 30–80°. These reflections were indexed as (111), (200), (220) and (311) which are interpreted for the structure of metallic silver and they are in accordance to the Joint Committee on Powder Diffraction Standards (JCPDS, file no. 04–0783). In XRD profile, the diffraction peak of (111) appears to be predominant peak (Fig. 7) along with two unknown peaks originated from the analyzed sample. It reveals the pure crystalline silver nitrate of synthesized AgNPs. These sharp Bragg peaks might have resulted due to the capping agent which is stabilizing the nanoparticle. These findings of obtaining Braggs reflection planes at resulted diffraction peaks for AgNPs are reported [1,19,20–21]. The XRD results also sug- gested that the crystallization of the bio-organic phase occurs on the surface of the silver nanoparticles. 5.5. FTIR — analysis FTIR measurements were taken to identify the nature of possible biomolecules responsible for surface coating and efficient stabilization of the AgNPs synthesized in E. scaber leaf extract. The FTIR spectra of leaf extract of E. scaber and the synthesized AgNPs were compared (Fig. 8). The broad peak around 3415 cm− 1 in the plant leaf extract is due to the stretching of vibration of functional groups. Results obtained from FTIR spectroscopic studies showed the chemical constituents in the leaf extract as well as synthesized AgNO3 particles with various peak values (Fig. 8). These variations are due to the stretching vibration in phenolics, amino acids, aliphatic and aromatic hydroxyl groups. The peaks at 2865 and 2933 cm− 1 are due to alkanes groups [2, 24,]. The peaks in the 1611 to 1400 cm−1 range (1606, 1542, 1402 cm−1) are due to the presence of aromatic rings in the E. scaber leaf extract. The peak at 1109 cm− 1 is due to the OH groups. The appearance of peaks at 1109 corresponds to primary and secondary amides (N–H group) and 827 due to alkyl halides (Fig. 8). E. scaber has been evaluated by many workers for the presence of all these secondary metabolites [6,8, 22,and 23]. The attachment of these three functional groups to the synthesized AgNPs suggested the role of these phytoconstituents in encapsulation and reduction of metal ion in E. scaber leaf extract. The stretching vibrations are due to the shifting of aliphatic and aromatic OH groups mediated by phenolic compounds. S.N. Kharat, V.D. Mendhulkar / Materials Science and Engineering C 62 (2016) 719–724 723 Fig. 8. FTIR spectra of E. scaber leaf extract (curve A) and AgNPs (curve B). Sr. Samples no. 1 Aqueous plant extract 2 Synthesized AgNPs 3 Ascorbic acid (standard) Concentration in μg/ml 50 100 150 200 250 50 100 150 200 250 20–140 μg/ml Scavenging ability in % 13.03 ± 0.08 40.73 ± 0.03 55.92 ± 0.02 67.62 ± 0.04 72.80 ± 0.07 15.23 ± 0.04 46.25 ± 0.09 60.15 ± 0.09 71.23 ± 0.05 85.90 ± 0.08 86.00 ± 0.40 IC50 value in μg/ml 113.6 ± 0.08 126.6 ± 0.06 41.86 ± 0.00 [Data are presented as mean ± SD (P b 0.05). IC50: Concentration of the aqueous extract and synthesized nanoparticles causing 50% DPPH radical scavenging ability].PDF Image | Antioxidant activity of Silver Nanoparticles using leaf extract
PDF Search Title:
Antioxidant activity of Silver Nanoparticles using leaf extractOriginal File Name Searched:
Synthesis_Characterization_and_studies_on_Antioxid.pdfDIY PDF Search: Google It | Yahoo | Bing
Turbine and System Plans CAD CAM: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. More Info
Waste Heat Power Technology: Organic Rankine Cycle uses waste heat to make electricity, shaft horsepower and cooling. More Info
All Turbine and System Products: Infinity Turbine ORD systems, turbine generator sets, build plans and more to use your waste heat from 30C to 100C. More Info
CO2 Phase Change Demonstrator: CO2 goes supercritical at 30 C. This is a experimental platform which you can use to demonstrate phase change with low heat. Includes integration area for small CO2 turbine, static generator, and more. This can also be used for a GTL Gas to Liquids experimental platform. More Info
Introducing the Infinity Turbine Products Infinity Turbine develops and builds systems for making power from waste heat. It also is working on innovative strategies for storing, making, and deploying energy. More Info
Need Strategy? Use our Consulting and analyst services Infinity Turbine LLC is pleased to announce its consulting and analyst services. We have worked in the renewable energy industry as a researcher, developing sales and markets, along with may inventions and innovations. More Info
Made in USA with Global Energy Millennial Web Engine These pages were made with the Global Energy Web PDF Engine using Filemaker (Claris) software.
Infinity Turbine Developing Spinning Disc Reactor SDR or Spinning Disc Reactors reduce processing time for liquid production of Silver Nanoparticles.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |