
PDF Publication Title:
Text from PDF Page: 010
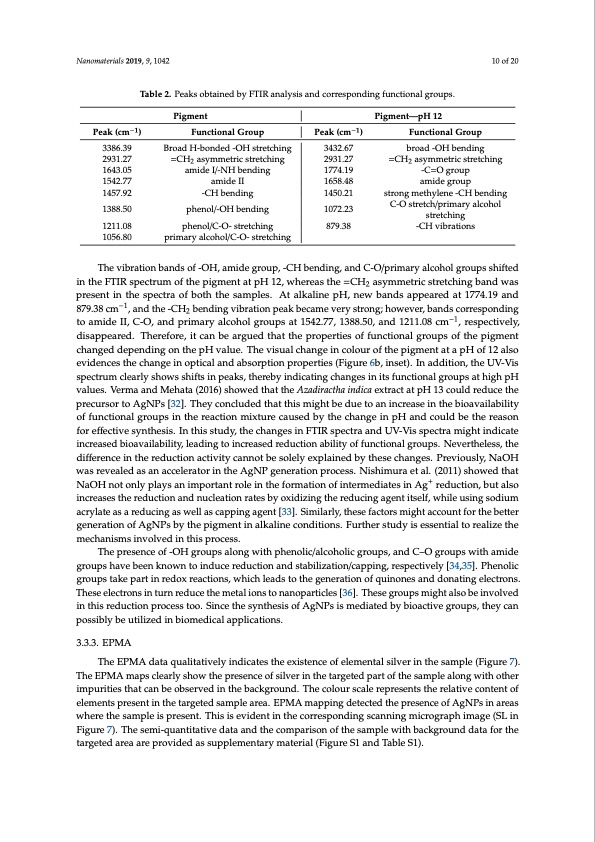
Nanomaterials 2019, 9, 1042 10 of 20 Table 2. Peaks obtained by FTIR analysis and corresponding functional groups. Peak (cm−1) 3386.39 2931.27 1643.05 1542.77 1457.92 1388.50 1211.08 1056.80 Pigment Functional Group Broad H-bonded -OH stretching =CH2 asymmetric stretching amide I/-NH bending amide II -CH bending phenol/-OH bending phenol/C-O- stretching primary alcohol/C-O- stretching Peak (cm−1) 3432.67 2931.27 1774.19 1658.48 1450.21 1072.23 879.38 Pigment—pH 12 Functional Group broad -OH bending =CH2 asymmetric stretching -C=O group amide group strong methylene -CH bending C-O stretch/primary alcohol stretching -CH vibrations The vibration bands of -OH, amide group, -CH bending, and C-O/primary alcohol groups shifted in the FTIR spectrum of the pigment at pH 12, whereas the =CH2 asymmetric stretching band was present in the spectra of both the samples. At alkaline pH, new bands appeared at 1774.19 and 879.38 cm−1, and the -CH2 bending vibration peak became very strong; however, bands corresponding to amide II, C-O, and primary alcohol groups at 1542.77, 1388.50, and 1211.08 cm−1, respectively, disappeared. Therefore, it can be argued that the properties of functional groups of the pigment changed depending on the pH value. The visual change in colour of the pigment at a pH of 12 also evidences the change in optical and absorption properties (Figure 6b, inset). In addition, the UV-Vis spectrum clearly shows shifts in peaks, thereby indicating changes in its functional groups at high pH values. Verma and Mehata (2016) showed that the Azadiractha indica extract at pH 13 could reduce the precursor to AgNPs [32]. They concluded that this might be due to an increase in the bioavailability of functional groups in the reaction mixture caused by the change in pH and could be the reason for effective synthesis. In this study, the changes in FTIR spectra and UV-Vis spectra might indicate increased bioavailability, leading to increased reduction ability of functional groups. Nevertheless, the difference in the reduction activity cannot be solely explained by these changes. Previously, NaOH was revealed as an accelerator in the AgNP generation process. Nishimura et al. (2011) showed that NaOH not only plays an important role in the formation of intermediates in Ag+ reduction, but also increases the reduction and nucleation rates by oxidizing the reducing agent itself, while using sodium acrylate as a reducing as well as capping agent [33]. Similarly, these factors might account for the better generation of AgNPs by the pigment in alkaline conditions. Further study is essential to realize the mechanisms involved in this process. The presence of -OH groups along with phenolic/alcoholic groups, and C–O groups with amide groups have been known to induce reduction and stabilization/capping, respectively [34,35]. Phenolic groups take part in redox reactions, which leads to the generation of quinones and donating electrons. These electrons in turn reduce the metal ions to nanoparticles [36]. These groups might also be involved in this reduction process too. Since the synthesis of AgNPs is mediated by bioactive groups, they can possibly be utilized in biomedical applications. 3.3.3. EPMA The EPMA data qualitatively indicates the existence of elemental silver in the sample (Figure 7). The EPMA maps clearly show the presence of silver in the targeted part of the sample along with other impurities that can be observed in the background. The colour scale represents the relative content of elements present in the targeted sample area. EPMA mapping detected the presence of AgNPs in areas where the sample is present. This is evident in the corresponding scanning micrograph image (SL in Figure 7). The semi-quantitative data and the comparison of the sample with background data for the targeted area are provided as supplementary material (Figure S1 and Table S1).PDF Image | Biosynthesis of Silver Nanoparticles Talaromyces purpurogenus
PDF Search Title:
Biosynthesis of Silver Nanoparticles Talaromyces purpurogenusOriginal File Name Searched:
nanomaterials-09-01042.pdfDIY PDF Search: Google It | Yahoo | Bing
Turbine and System Plans CAD CAM: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. More Info
Waste Heat Power Technology: Organic Rankine Cycle uses waste heat to make electricity, shaft horsepower and cooling. More Info
All Turbine and System Products: Infinity Turbine ORD systems, turbine generator sets, build plans and more to use your waste heat from 30C to 100C. More Info
CO2 Phase Change Demonstrator: CO2 goes supercritical at 30 C. This is a experimental platform which you can use to demonstrate phase change with low heat. Includes integration area for small CO2 turbine, static generator, and more. This can also be used for a GTL Gas to Liquids experimental platform. More Info
Introducing the Infinity Turbine Products Infinity Turbine develops and builds systems for making power from waste heat. It also is working on innovative strategies for storing, making, and deploying energy. More Info
Need Strategy? Use our Consulting and analyst services Infinity Turbine LLC is pleased to announce its consulting and analyst services. We have worked in the renewable energy industry as a researcher, developing sales and markets, along with may inventions and innovations. More Info
Made in USA with Global Energy Millennial Web Engine These pages were made with the Global Energy Web PDF Engine using Filemaker (Claris) software.
Infinity Turbine Developing Spinning Disc Reactor SDR or Spinning Disc Reactors reduce processing time for liquid production of Silver Nanoparticles.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |