
PDF Publication Title:
Text from PDF Page: 009
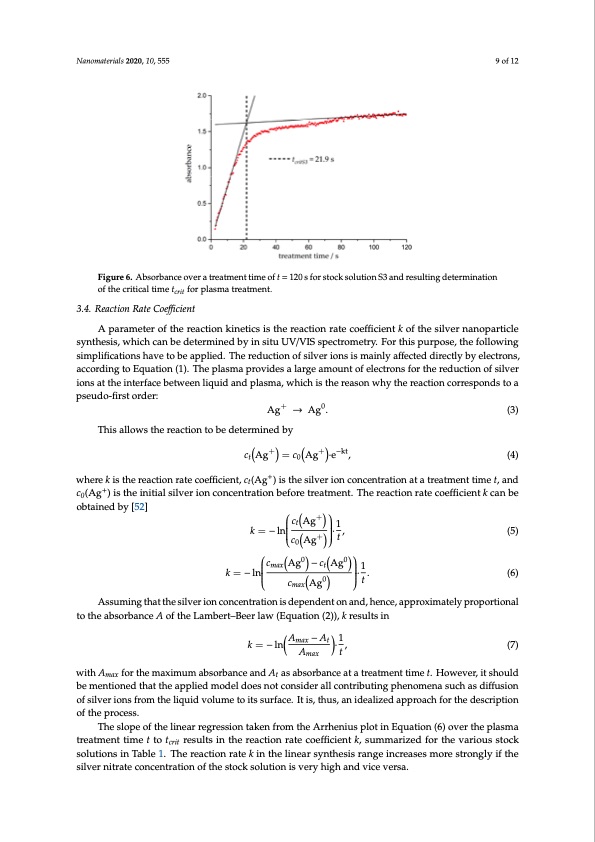
Nanomaterials 2020, 10, 555 9 of 12 Nanomaterials 2020, 10, 555 10 of 13 Figure 6. Absorbance over a treatment time of t = 120 s for stock solution S3 and resulting determination Figure 6. Absorbance over a treatment time of t = 120 s for stock solution S3 and resulting of the critical time t determination of the critical time tcrit for plasma treatment. obtained by [52] c Ag0 − c Ag0 1 max t crit 3.4. Reaction Rate Coefficient 3.4. Reaction Rate Coefficient for plasma treatment. A parameter of the reaction kinetics is the reaction rate coefficient k of the silver nanoparticle synthesis, which can be determined by in situ UV/VIS spectrometry. For this purpose, the following A parameter of the reaction kinetics is the reaction rate coefficient k of the silver nanoparticle simplifications have to be applied. The reduction of silver ions is mainly affected directly by electrons, synthesis, which can be determined by in situ UV/VIS spectrometry. For this purpose, the following according to Equation (1). The plasma provides a large amount of electrons for the reduction of silver simplificiaotnisoantsthhe ainvterfatoce betwaepenpliqeudid. aTnhdeplraesmdua,cwtihoinch iosfthseilrveaesroniownhsy tihse rmeaacitniolnycoarfrfeespctoends dtoiraectly by electrons, according to Equation (1). The plasma provides a large amount of electrons for the pseudo-first order: This allows the reaction to be determined by +0 Ag →Ag. (3) reduction of silver ions at the interface between liquid and plasma, which is the reason why the reaction corresponds to a pseudo-first order: + + +0−kt cAgA=gc→AAgg·e., (4) (3) + Thiswahlelroewk sistherreeaactciotinornateocobeffidceietnetr,mc (iAnged) ibsythe silver ion concentration at a treatment time t, and t0 t c (Ag+) is the initial silver ion concentration before treatment. The reaction rate coefficient k can be 0 obtained by [52] 𝑐 Ag+ = 𝑐 Ag+ ∙ e-kt, (4) + + ctAg 1 where k is the reaction rate coefficient, ct(Ag ) is the silver ion concentration at a treatment time t, and k=−ln · , (5) c Ag+ t + c0(Ag ) is the initial silver ion concentration befor0e treatment. The reaction rate coefficient k can be k=−ln ·. + (6) 𝑐Ag 1 cAg0 t 𝑘=-ln ∙ , 𝑐 Ag+ 𝑡 (5) ∙ . (6) max Assuming that the silver ion concentration is dependent on and, hence, approximately proportional to the absorbance A of the Lambert–Beer law (Equation (2)), k results in 𝑐 Ag0-𝑐Ag0 1 𝑡 𝑘=-ln max t k=−lnA −A0·1, (7) with A for the maximum absorbance and A as absorbance at a treatment time t. However, it should proportional to the absorbance A of the Lambert–Beer law (Equation (2)), k results in of silver ions from the liquid volume to its surface. It is, thus, an idealized approach for the description 𝑐 A A g t max Assumingmaxthat the silver ion concentrtation is dependent on and, hence, approximately be mentioned that the applied model does not consider all contributing phenomena such as diffusion of the process. 𝑘 = -ln 𝐴 − 𝐴 ∙ 1, (7) 𝐴𝑡 The slope of the linear regression taken from the Arrhenius plot in Equation (6) over the plasma treatment time t to tcrit results in the reaction rate coefficient k, summarized for the various stock with Amax for the maximum absorbance and At as absorbance at a treatment time t. However, it solutions in Table 1. The reaction rate k in the linear synthesis range increases more strongly if the should bseilvmerenitiroatneecdontcheantrtahtieonaopfpthliesdtomckosdoleultidonoeisvneoryt hciognhsainddevriacellvceorsnat. ributing phenomena such as diffusion of silver ions from the liquid volume to its surface. It is, thus, an idealized approach for the description of the process. The slope of the linear regression taken from the Arrhenius plot in Equation (6) over the plasma treatment time t to tcrit results in the reaction rate coefficient k, summarized for the various stock solutions in Table 1. The reaction rate k in the linear synthesis range increases more strongly if thePDF Image | Formation Kinematics of Plasma-Generated Silver Nanoparticles
PDF Search Title:
Formation Kinematics of Plasma-Generated Silver NanoparticlesOriginal File Name Searched:
nanomaterials-10-00555-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Turbine and System Plans CAD CAM: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. More Info
Waste Heat Power Technology: Organic Rankine Cycle uses waste heat to make electricity, shaft horsepower and cooling. More Info
All Turbine and System Products: Infinity Turbine ORD systems, turbine generator sets, build plans and more to use your waste heat from 30C to 100C. More Info
CO2 Phase Change Demonstrator: CO2 goes supercritical at 30 C. This is a experimental platform which you can use to demonstrate phase change with low heat. Includes integration area for small CO2 turbine, static generator, and more. This can also be used for a GTL Gas to Liquids experimental platform. More Info
Introducing the Infinity Turbine Products Infinity Turbine develops and builds systems for making power from waste heat. It also is working on innovative strategies for storing, making, and deploying energy. More Info
Need Strategy? Use our Consulting and analyst services Infinity Turbine LLC is pleased to announce its consulting and analyst services. We have worked in the renewable energy industry as a researcher, developing sales and markets, along with may inventions and innovations. More Info
Made in USA with Global Energy Millennial Web Engine These pages were made with the Global Energy Web PDF Engine using Filemaker (Claris) software.
Infinity Turbine Developing Spinning Disc Reactor SDR or Spinning Disc Reactors reduce processing time for liquid production of Silver Nanoparticles.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |