
PDF Publication Title:
Text from PDF Page: 005
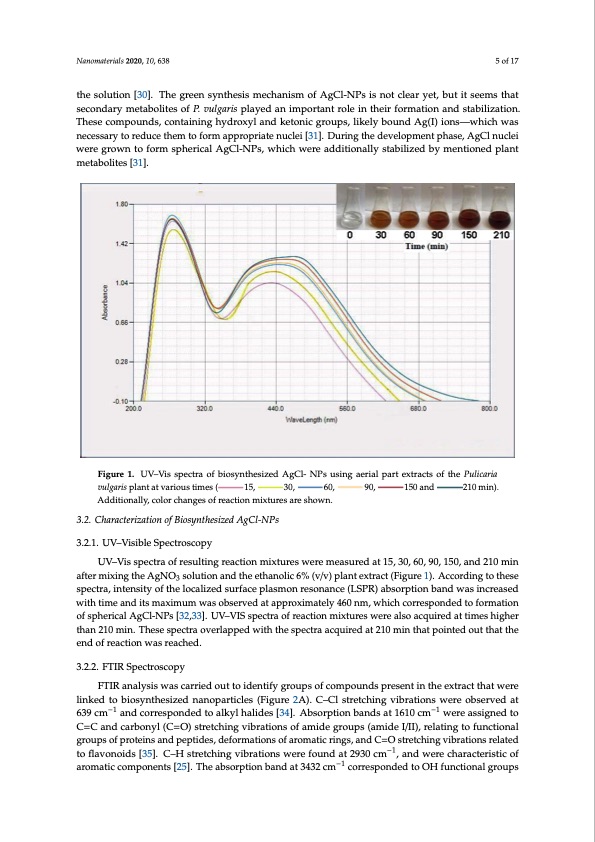
Nanomaterials 2020, 10, x FOR PEER REVIEW 5 of 17 NNaNnanonmomamtaeatreteteiereraririilaiasallsl2ls202020,01,,,010,0x,,,xFxFOFORORPRPEPERERREREVEVIVEIEIEIWEW 55o5ofof1f717 take place, and the colorless AgNO3 solution became dark brown with time (Figure 1). This is likely tatatakakekepplpalalacacece,e,a,anandndthththeheccocolololorolrerlelesesAsAggNgNOO3 3s3sosoloululutuitotitioiononbbebececacamameeddadarakrkbbrborowownnwitihitiththtitmtitimimee(F(F(FiFgigiguguruere11)1.).).T.ThThihsisisissisislsilklilikikekeleylyly attributable to the completion of the synthesis process, and the formation of biogenic AgCl-NPs [11]. aatatrtirtrbNribibubabunutuoatmtatababablbteleleleretiaotltotosot2ht0ththeh2he0ec,coc1com0om,m6p3plp8peleleteitotitioinonoofoftfhththehessysynyntnhththehesesisisispsprporococeceses,s,a,anandndthththehefofoforomrmaataitotitioiononoofofbfbiboioiogogegenenincicicAcAggCgClC-lNl-l-N-NPPsPs[s1[1[151]1.]o].].f.17 It was previously reported that P. vulgaris contains alkaloids, tannins, flavonoids, and phenolic ItItItwaasaspprperevevivoioiouoususlsyslylyrerepepoporotrertetededthththahatatPP.P..v.vuvuluglglglagarairsrisisisccoconontnatataianininsnsaalaklklkakalaololoiodididsds,s,,t,atatananinininsns,s,,f,lfaflflalavavovonononoiodididsds,s,,a, anandndpphphehenenonoloilclilicic compounds [29] that could be responsible for the bioreduction of Ag(I) ions into AgCl-NPs as well ccocomomppopouounundndsds[s2[2[2929]9]t]hththahatatctcocououludldldbbebereresespspopononsnsisbsibiblbelelefofoforortrhththehebbiboioioroerededuducuctcitotitioiononoofofAfAgg(gI()(I(I)I)i)oioiononsnsisninintnototoAAggCgClC-lNl-l-N-NPPsPsasasaswsweelel the solution [30]. The green synthesis mechanism of AgCl-NPs is not clear yet, but it seems that as their capping in the solution [30]. The green synthesis mechanism of AgCl-NPs is not clear yet, aasasthththeheierirircrcacapapipniningnginininthththehessosoloululutuitotitioionon[3[3[3030]0.].].T.ThTheheggrgerenenssysynyntnhththehesesisisismeecechchahananinsismismoofofAfAggCgClC-lNl-l-N-NPPsPsisisisnnonototctclcecleleaeararyryeyete,t,t,, secondary metabolites of P. vulgaris played an important role in their formation and stabilization. but it seems that secondary metabolites of P. vulgaris played an important role in their formation bbubututittititstsesememssthththahatatstsesececoconondndadararyrymeeteatatababoboloiltlilieititetesesoofofPfP.P.v.vuvuluglglglagarairsrisisispplpalalayayeyededaananimimimppoporotrartatanantntrtoroloeleleinininthththeheieririrfofoforomrmaataitotitioionon These compounds, containing hydroxyl and ketonic groups, likely bound Ag(I) ions—which was and stabilization. These compounds, containing hydroxyl and ketonic groups, likely bound Ag(I) aanandndsstsatatababiblililzlilizizazataitotitioionon.n..T.ThTheheseseseccocomomppopouounundndsds,s,,c,coconontnatataianininininingnghhyhydydrdroroxoxyxylylalanandndkkeketeototononincicicggrgorououpupsps,s,,l,ilklilikiekeleylylybbobouounundndAAgg(gI()(I(I)I) necessary to reduce them to form appropriate nuclei [31]. During the development phase, AgCl nuclei ions—which was necessary to reduce them to form appropriate nuclei [31]. During the ioioiononsns—s—whhihcicihchwaasasnnenecececesesasarayrytototorerededuducucecethththehememtototofofoforomrmaapaprporopoprpirariaiataetetennunucuclceleleiei[3[3[3131]1.].]..DDuuruirnriningngthththehe were grown to form spherical AgCl-NPs, which were additionally stabilized by mentioned plant development phase, AgCl nuclei were grown to form spherical AgCl-NPs, which were additionally ddedeveveveleololopopmpmeenentntptphphahasasese,e,A,AggCgClClnlnunucuclceleleieiwiweerereggrgorowownntototofofoforomrmsspsphphehereircriciacalalAlAggCgClC-lNl-l-N-NPPsPs,s,w,whhihcicihchweerereaadadidtititotitioiononanalalylyly metabolites [31]. stabilized by mentioned plant metabolites [31]. sstsatatababiblililzlilizizezededbbybymeenentnitotitioiononenededpplpalalnantntmtmeeteatatababoboloiltlilieititeteses[s3[3[3131]1.].].. Figure 1. UV–Vis spectra of biosynthesized AgCl- NPs using aerial part extracts of the Pulicaria FFiFgigigugurFuerirege1u1.1r1.e.U. UV1UV.V–V–V–U–ViVsVisisis–sVpspeipsecetcscrctptatrtraeaocotforfabf biboioioisfoysbsynyintonhtsthtehyheseneiszstsihzizeizededsdiAzAeAgAdgCgClCA-l-l-lg-N-CNPlP-sPsNsuusuPuisnsininignungsgaiaenaeregeirariaialiaelplrpaiparalatrtptet eaxexrxtxrtatrtreracaxtcsctrtstsaoocofotfstfhthohtehfePtPhuPueluilcliliPliacicicrauairlrariiaicaiaria vulgaris plant at various times ( 15, 30, 60, 90, vvuvulugllglgalgvgrauairsrlriigsisispaprlpailslalanlapntnltatnatatvtavavtvaravairoarioioiuroiusuousustistmtititmimimimemeseses(s( 11515,5,, 33030,0, 66060,0,, 99090,0,, min). Additionally, color changes of reaction mixtures are shown. mmininini)nA.).).A.d.AdAddidtdididtoititinotitioioinaonlanlnalyalal,lylcylyl,yoy,c,,l,ococolorololoclrohrcrahcnhahganaengnsgegoesesfsosrofoeofrfafercreaeteaciaotcicntotitioioinmonmixmitxuixixtixrxutetutrusueraresersasesaraserhreseohswshohownow.wnn.n.. 150 and 210 15151505050aananandnd 21212021010m0in). UV–Vis spectra of resulting reaction mixtures were measured at 15, 30, 60, 90, 150, and 210 min UUVV–V–V–ViVsisisspspepecectcrtartraoofofrferesesusulutliltltntitiningngrereaeacactcitotitioiononmixixixtxututuruereseswsweereremeeaeasasusuruerededaatat1t1515,5,3,3030,0,6,6060,0,9,9090,0,1,1515050,0,a,anandnd22121010mininin 3.2. Characterization of Biosynthesized AgCl-NPs 3.2. Characterization of Biosynthesized AgCl-NPs 33.32..2.2.2.C.ChChahararacactcetreteirzriziazizataitotitioinoionoofofBfBiBoioisoioysynyntnhththehseiszsiziezizdedAAgAgCgClC-lN-l-l-N-NPPsPs 3.2.1. UV–Visible Spectroscopy 3.2.1. UV–visible Spectroscopy 33.32..2.2.21..1.1.1.U.UVV–V–v–vivsisisbsibiblbeleleSeSpSpepecectcrtortrososcscocopopypy UV–Vis spectra of resulting reaction mixtures were measured at 15, 30, 60, 90, 150, and 210 min after mixing the AgNO3 solution and the ethanolic 6% (v/v) plant extract (Figure 1). According to these after mixing the AgNO3 solution and the ethanolic 6% (v/v) plant extract (Figure 1). According to aafatfeftfteterermixixixixniningngthththeheAAggNgNOO3 3s3sosoloululutuitotitioinonaanandndthththeheetehththahananonoloilclilicic66%6%(v(v(v/v/v/v)v)p)plpalalanantntetexextxrtratracactct(tF(F(FiFgigiguguruere11)1.).)..A.Accocorodrdidniningngtototo spectra, intensity of the localized surface plasmon resonance (LSPR) absorption band was increased these spectra, intensity of the localized surface plasmon resonance (LSPR) absorption band was thththehesesesesspspepecectcrtratra,a,,i,ninintnetetenensnsistsiyititytyoofofthththehelololococacalailzlilizizezededssusurufrarfafacacecepplpalalasasmsmoononreresesosononananancncece(L(LSLSPSPRPR)R)aababsbsosoroprptpitotitioiononbbabanandndwaasas with time and its maximum was observed at approximately 460 nm, which corresponded to formation increased with time and its maximum was observed at approximately 460 nm, which corresponded ininincncrcereaeasasesededwitihitiththtitmtitimimeeaanandnditisititstsmsmaaxaxixmimimuumumwaasasosobobsbseserevrvevededaatatatapaprporoxoxixmimimaataeteteleylyly44646060nnmnm, ,w,whhihcicihchccocororeresespspoponondndededed of spherical AgCl-NPs [32,33]. UV–VIS spectra of reaction mixtures were also acquired at times higher to formation of spherical AgCl-NPs [32,33]. UV–VIS spectra of reaction mixtures were also acquired tototofofoforomrmaataitotitioiononoofofsfspsphphehereircriciacalalAlAggCgClC-lNl-l-N-NPPsPs[s3[3[3232,23,,3,3,3]3.].].U.UVV–V–V–VIVSISISsspspepecectcrtartraoofofrfereaeacactcitotitioiononmixixixtxututuruereseswsweerereaalaslsolsoaacacqcququiurierireded than 210 min. These spectra overlapped with the spectra acquired at 210 min that pointed out that the at times higher than 210 min. These spectra overlapped with the spectra acquired at 210 min that aatatitmtitimimeeseshhihgigighghehererthththahanan22121010mininin.n..T.ThThehesesesesspspepecectcrtartraoovoveverelrarlalapapepededwitihitithththththehesspspepecectcrtartraacacqcququiurierirededaatat2t2121010minininthththahatat end of reaction was reached. pointed out that the end of reaction was reached. ppopoioninintnetetededoouoututhththahatathththeheenendndoofofrfereaeacactcitotitioiononwaasasrsereaeacachcheheded.d.. 3.2.2. FTIR Spectroscopy 3.2.2. FTIR Spectroscopy 33.32..2.2.2..2.2.2.F.FTFTITRIRIRSSpSpepecectcrtortrososcscocopopypy FTIR analysis was carried out to identify groups of compounds present in the extract that were FTIR analysis was carried out to identify groups of compounds present in the extract that were FFTFTITRIRIRaanananalaylylysysisisiswswaasascscacararirerieiededoouoututototoidididedenentnitftitiyififyfyggrgorououpupspsosofofcfcocomomppopouounundndsdspsprperesesesenentntitnininthththeheexextxrtartracactcthththahatatwtweerere linked to biosynthesized nanoparticles (Figure 2A). C–Cl stretching vibrations were observed at linked to biosynthesized nanoparticles (Figure 2A). C–Cl stretching vibrations were observed at 639 lilnlilininknkekededtototobbib−oioi1sosysynyntnhththehesesiszsizizezedednnanananonopopaparatrirtctiticilceleleses(sF(F(FiFgigiguguruere22A2A).).).C.C–C–C–ClClslstsrtertretectcthchihniningngvvivbibibrbarataitotitioiononsnswsweerere−oo1bobsbseserevrvevededaatat6t6363939 639 cm and corresponded to alkyl halides [34]. Absorption bands at 1610 cm were assigned to −1−c−1−1m1 −1 and corresponded to alkyl halides [34]. Absorption bands at 1610−1−c−1−1m1 −1 were assigned to C=C ccmcm aanandndccocororeresespspoponondndedededtototoaalaklklkykylylhlhahalaildlilididedeses[3[3[3434]4.].]..A.Abbsbsosoroprptpitotitioinonbbabanandndsdsaatat1t1616161010ccmcm weererereaasasisgsigigngnenededtototoCC=C=C=C C=C and carbonyl (C=O) stretching vibrations of amide groups (amide I/II), relating to functional and carbonyl (C=O) stretching vibrations of amide groups (amide I/II), relating to functional groups aanandndccacararbrbobononynylyl(lC(C(C=C=O=O))s)stsrtertretectcthchihniningngvvivbibibrbarataitotitioiononsnsosofofafamamidididedeggrgorououpupsps(sa(a(amamidididedeI/I/I/I/I/I)I,I)I),r,erelealalataitntitiningngtototofufufununcnctcitotitioiononanalalglgrgorououpupsps groups of proteins and peptides, deformations of aromatic rings, and C=O stretching vibrations related to flavonoids [35]. C–H stretching vibrations were found at 2930 cm−1, and were characteristic of aromatic components [25]. The absorption band at 3432 cm−1 corresponded to OH functional groupsPDF Image | Synthesis of Biogenic Silver Nanoparticles Aerial Part Extract
PDF Search Title:
Synthesis of Biogenic Silver Nanoparticles Aerial Part ExtractOriginal File Name Searched:
nanomaterials-10-00638.pdfDIY PDF Search: Google It | Yahoo | Bing
Turbine and System Plans CAD CAM: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. More Info
Waste Heat Power Technology: Organic Rankine Cycle uses waste heat to make electricity, shaft horsepower and cooling. More Info
All Turbine and System Products: Infinity Turbine ORD systems, turbine generator sets, build plans and more to use your waste heat from 30C to 100C. More Info
CO2 Phase Change Demonstrator: CO2 goes supercritical at 30 C. This is a experimental platform which you can use to demonstrate phase change with low heat. Includes integration area for small CO2 turbine, static generator, and more. This can also be used for a GTL Gas to Liquids experimental platform. More Info
Introducing the Infinity Turbine Products Infinity Turbine develops and builds systems for making power from waste heat. It also is working on innovative strategies for storing, making, and deploying energy. More Info
Need Strategy? Use our Consulting and analyst services Infinity Turbine LLC is pleased to announce its consulting and analyst services. We have worked in the renewable energy industry as a researcher, developing sales and markets, along with may inventions and innovations. More Info
Made in USA with Global Energy Millennial Web Engine These pages were made with the Global Energy Web PDF Engine using Filemaker (Claris) software.
Infinity Turbine Developing Spinning Disc Reactor SDR or Spinning Disc Reactors reduce processing time for liquid production of Silver Nanoparticles.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |